Immunoexpression Pattern of Autophagy-Related Proteins in Human Congenital Anomalies of the Kidney and Urinary Tract
- PMID: 38999938
- PMCID: PMC11241479
- DOI: 10.3390/ijms25136829
Immunoexpression Pattern of Autophagy-Related Proteins in Human Congenital Anomalies of the Kidney and Urinary Tract
Abstract
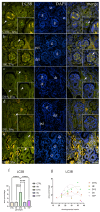
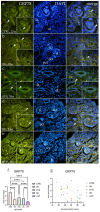
The purpose of this study was to evaluate the spatiotemporal immunoexpression pattern of microtubule-associated protein 1 light chain 3 beta (LC3B), glucose-regulated protein 78 (GRP78), heat shock protein 70 (HSP70), and lysosomal-associated membrane protein 2A (LAMP2A) in normal human fetal kidney development (CTRL) and kidneys affected with congenital anomalies of the kidney and urinary tract (CAKUT). Human fetal kidneys (control, horseshoe, dysplastic, duplex, and hypoplastic) from the 18th to the 38th developmental week underwent epifluorescence microscopy analysis after being stained with antibodies. Immunoreactivity was quantified in various kidney structures, and expression dynamics were examined using linear and nonlinear regression modeling. The punctate expression of LC3B was observed mainly in tubules and glomerular cells, with dysplastic kidneys displaying distinct staining patterns. In the control group's glomeruli, LAMP2A showed a sporadic, punctate signal; in contrast to other phenotypes, duplex kidneys showed significantly stronger expression in convoluted tubules. GRP78 had a weaker expression in CAKUT kidneys, especially hypoplastic ones, while normal kidneys exhibited punctate staining of convoluted tubules and glomeruli. HSP70 staining varied among phenotypes, with dysplastic and hypoplastic kidneys exhibiting stronger staining compared to controls. Expression dynamics varied among observed autophagy markers and phenotypes, indicating their potential roles in normal and dysfunctional kidney development.
Keywords: CAKUT; GRP78; HSP70; LAMP2A; LC3B; autophagy; congenital anomalies of the kidney and urinary tract; nephrogenesis.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, nor in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Saran R., Robinson B., Abbott K.C., Bragg-Gresham J., Chen X., Gipson D., Gu H., Hirth R.A., Hutton D., Jin Y., et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020;75:A6–A7. doi: 10.1053/j.ajkd.2019.09.003. - DOI - PubMed
-
- Xie Y., Bowe B., Mokdad A.H., Xian H., Yan Y., Li T., Maddukuri G., Tsai C.Y., Floyd T., Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

