Genetic Analysis of SCN11A, SCN10A, and SCN9A in Familial Episodic Pain Syndrome (FEPS) in Japan and Proposal of Clinical Diagnostic Criteria
- PMID: 38999942
- PMCID: PMC11241565
- DOI: 10.3390/ijms25136832
Genetic Analysis of SCN11A, SCN10A, and SCN9A in Familial Episodic Pain Syndrome (FEPS) in Japan and Proposal of Clinical Diagnostic Criteria
Abstract
Familial episodic pain syndrome (FEPS) is an early childhood onset disorder of severe episodic limb pain caused mainly by pathogenic variants of SCN11A, SCN10A, and SCN9A, which encode three voltage-gated sodium channels (VGSCs) expressed as key determinants of nociceptor excitability in primary sensory neurons. There may still be many undiagnosed patients with FEPS. A better understanding of the associated pathogenesis, epidemiology, and clinical characteristics is needed to provide appropriate diagnosis and care. For this study, nationwide recruitment of Japanese patients was conducted using provisional clinical diagnostic criteria, followed by genetic testing for SCN11A, SCN10A, and SCN9A. In the cohort of 212 recruited patients, genetic testing revealed that 64 patients (30.2%) harbored pathogenic or likely pathogenic variants of these genes, consisting of 42 (19.8%), 14 (6.60%), and 8 (3.77%) patients with variants of SCN11A, SCN10A, and SCN9A, respectively. Meanwhile, the proportions of patients meeting the tentative clinical criteria were 89.1%, 52.0%, and 54.5% among patients with pathogenic or likely pathogenic variants of each of the three genes, suggesting the validity of these clinical criteria, especially for patients with SCN11A variants. These clinical diagnostic criteria of FEPS will accelerate the recruitment of patients with underlying pathogenic variants who are unexpectedly prevalent in Japan.
Keywords: SCN10A; SCN11A; SCN9A; familial episodic pain syndrome.
Conflict of interest statement
The authors declare the following conflicts of interest: Akio Koizumi, Hiroko Okuda, Tohru Tezuka, and Aya Takeya received personal fees from AlphaNavi Pharma during the study period. Hiroko Okuda and Tohru Tezuka received grants from AlphaNavi Pharma during the study period. Shohab Youssefian received funding from AlphaNavi Pharma. Atsuko Noguchi, Takeshi Yoshida, Shinji Akioka, Takeshi Asano, Michimasa Fujiwara, Manabu Tanaka, Kaoru Eto, Hideaki Shiraishi, and Tsutomu Takahashi received trial compensation from Alpha Navi Pharma. Atsuko Noguchi, Tsutomu Takahashi, Akio Koizumi, Hatasu Kobayashi, and Kouji H. Harada have a patent regarding
Figures
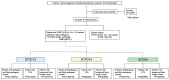
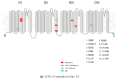


References
-
- Alsaloum M., Labau J.I.R., Sosniak D., Zhao P., Almomani R., Gerrits M., Hoeijmakers J.G.J., Lauria G., Faber C.G., Waxman S.G., et al. A novel gain-of-function sodium channel β2 subunit mutation in idiopathic small fiber neuropathy. J. Neurophysiol. 2021;126:827–839. doi: 10.1152/jn.00184.2021. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

