Inflammatory Changes after Medical Suppression of Suspected Endometriosis for Implantation Failure: Preliminary Results
- PMID: 38999962
- PMCID: PMC11241468
- DOI: 10.3390/ijms25136852
Inflammatory Changes after Medical Suppression of Suspected Endometriosis for Implantation Failure: Preliminary Results
Abstract
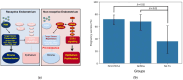
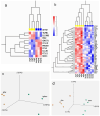
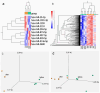
Unexplained euploid embryo transfer failure (UEETF) is a frustrating and unanswered conundrum accounting for 30 to 50% of failures in in vitro fertilization using preimplantation genetic testing for aneuploidy (PGT-A). Endometriosis is thought by many to account for most of such losses and menstrual suppression or surgery prior to the next transfer has been reported to be beneficial. In this study, we performed endometrial biopsy in a subset of women with UEETF, testing for the oncogene BCL6 and the histone deacetylase SIRT1. We compared 205 PGT-A cycles outcomes and provide those results following treatment with GnRH agonist versus controls (no treatment). Based on these and previous promising results, we next performed a pilot randomized controlled trial comparing the orally active GnRH antagonist, elagolix, to oral contraceptive pill (OCP) suppression for 2 months before the next euploid embryo transfer, and monitored inflammation and miRNA expression in blood, before and after treatment. These studies support a role for endometriosis in UEETF and suggest that medical suppression of suspected disease with GnRH antagonist prior to the next transfer could improve success rates and address underlying inflammatory and epigenetic changes associated with UEETF.
Keywords: endometriosis; implantation; infertility; inflammation; recurrent pregnancy loss.
Conflict of interest statement
The authors declare no conflicts of interest, with the exception of Dan Angress who was PI for the NIH R44 funding and is Chief Operating Officer (COO) of CiceroDx, Inc. Bruce Lessey is associated with licensed technology from Prisma Health, related to BCL6 and SIRT1 biomarkers. Daniel Angress was employed in CiceroDx. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

