Idiosyncratic Drug-Induced Liver Injury and Amoxicillin-Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients
- PMID: 38999973
- PMCID: PMC11241776
- DOI: 10.3390/ijms25136863
Idiosyncratic Drug-Induced Liver Injury and Amoxicillin-Clavulanate: Spotlight on Gut Microbiota, Fecal Metabolome and Bile Acid Profile in Patients
Abstract
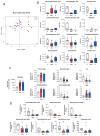
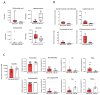
Several hepatic disorders are influenced by gut microbiota, but its role in idiosyncratic drug-induced liver injury (iDILI), whose main causative agent is amoxicillin-clavulanate, remains unknown. This pioneering study aims to unravel particular patterns of gut microbiota composition and associated metabolites in iDILI and iDILI patients by amoxicillin-clavulanate (iDILI-AC). Thus, serum and fecal samples from 46 patients were divided into three study groups: healthy controls (n = 10), non-iDILI acute hepatitis (n = 12) and iDILI patients (n = 24). To evaluate the amoxicillin-clavulanate effect, iDILI patients were separated into two subgroups: iDILI non-caused by amoxicillin-clavulanate (iDILI-nonAC) (n = 18) and iDILI-AC patients (n = 6). Gut microbiota composition and fecal metabolome plus serum and fecal bile acid (BA) analyses were performed, along with correlation analyses. iDILI patients presented a particular microbiome profile associated with reduced fecal secondary BAs and fecal metabolites linked to lower inflammation, such as dodecanedioic acid and pyridoxamine. Moreover, certain taxa like Barnesiella, Clostridia UCG-014 and Eubacterium spp. correlated with significant metabolites and BAs. Additionally, comparisons between iDILI-nonAC and iDILI-AC groups unraveled unique features associated with iDILI when caused by amoxicillin-clavulanate. In conclusion, specific gut microbiota profiles in iDILI and iDILI-AC patients were associated with particular metabolic and BA status, which could affect disease onset and progression.
Keywords: amoxicillin–clavulanate; bile acids; drug-induced liver disease; fecal metabolome; gut microbiota.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Impact of Amoxicillin-Clavulanate followed by Autologous Fecal Microbiota Transplantation on Fecal Microbiome Structure and Metabolic Potential.mSphere. 2018 Nov 21;3(6):e00588-18. doi: 10.1128/mSphereDirect.00588-18. mSphere. 2018. PMID: 30463925 Free PMC article.
-
Comprehensive Analysis of Serum and Fecal Bile Acid Profiles and Interaction with Gut Microbiota in Primary Biliary Cholangitis.Clin Rev Allergy Immunol. 2020 Feb;58(1):25-38. doi: 10.1007/s12016-019-08731-2. Clin Rev Allergy Immunol. 2020. PMID: 30900136
-
Fecal microbiome and bile acid metabolome in adult short bowel syndrome.Am J Physiol Gastrointest Liver Physiol. 2022 Jan 1;322(1):G154-G168. doi: 10.1152/ajpgi.00091.2021. Epub 2021 Nov 24. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 34816756 Free PMC article.
-
The chemical, genetic and immunological basis of idiosyncratic drug-induced liver injury.Hum Exp Toxicol. 2015 Dec;34(12):1310-7. doi: 10.1177/0960327115606529. Hum Exp Toxicol. 2015. PMID: 26614821 Review.
-
Mechanisms of idiosyncratic drug-induced liver injury.Adv Pharmacol. 2019;85:133-163. doi: 10.1016/bs.apha.2018.12.001. Epub 2019 Jan 18. Adv Pharmacol. 2019. PMID: 31307585 Review.
Cited by
-
Susceptibility Factor TNF-α Synergizes with Polygonum multiflorum to Drive Idiosyncratic Liver Injury in Mice by Disrupting Gut Microbiota Composition and Hepatic Metabolite Homeostasis.J Inflamm Res. 2025 Jul 17;18:9477-9494. doi: 10.2147/JIR.S528667. eCollection 2025. J Inflamm Res. 2025. PMID: 40692546 Free PMC article.
-
Gut microbiota in liver diseases: initiation, development and therapy.Front Med (Lausanne). 2025 Jun 4;12:1615839. doi: 10.3389/fmed.2025.1615839. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40534699 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

