Constructing Direct Z-Scheme Y2TmSbO7/GdYBiNbO7 Heterojunction Photocatalyst with Enhanced Photocatalytic Degradation of Acetochlor under Visible Light Irradiation
- PMID: 38999979
- PMCID: PMC11241117
- DOI: 10.3390/ijms25136871
Constructing Direct Z-Scheme Y2TmSbO7/GdYBiNbO7 Heterojunction Photocatalyst with Enhanced Photocatalytic Degradation of Acetochlor under Visible Light Irradiation
Abstract
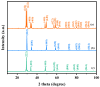
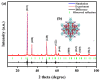
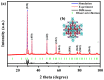
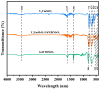
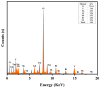
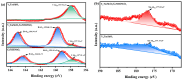
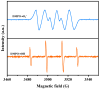
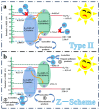
This study presents a pioneering synthesis of a direct Z-scheme Y2TmSbO7/GdYBiNbO7 heterojunction photocatalyst (YGHP) using an ultrasound-assisted hydrothermal synthesis technique. Additionally, novel photocatalytic nanomaterials, namely Y2TmSbO7 and GdYBiNbO7, were fabricated via the hydrothermal fabrication technique. A comprehensive range of characterization techniques, including X-ray diffractometry, Fourier-transform infrared spectroscopy, Raman spectroscopy, UV-visible spectrophotometry, X-ray photoelectron spectroscopy, transmission electron microscopy, X-ray energy-dispersive spectroscopy, fluorescence spectroscopy, photocurrent testing, electrochemical impedance spectroscopy, ultraviolet photoelectron spectroscopy, and electron paramagnetic resonance, was employed to thoroughly investigate the morphological features, composition, chemical, optical, and photoelectric properties of the fabricated samples. The photocatalytic performance of YGHP was assessed in the degradation of the pesticide acetochlor (AC) and the mineralization of total organic carbon (TOC) under visible light exposure, demonstrating eximious removal efficiencies. Specifically, AC and TOC exhibited removal rates of 99.75% and 97.90%, respectively. Comparative analysis revealed that YGHP showcased significantly higher removal efficiencies for AC compared to the Y2TmSbO7, GdYBiNbO7, or N-doped TiO2 photocatalyst, with removal rates being 1.12 times, 1.21 times, or 3.07 times higher, respectively. Similarly, YGHP demonstrated substantially higher removal efficiencies for TOC than the aforementioned photocatalysts, with removal rates 1.15 times, 1.28 times, or 3.51 times higher, respectively. These improvements could be attributed to the Z-scheme charge transfer configuration, which preserved the preferable redox capacities of Y2TmSbO7 and GdYBiNbO7. Furthermore, the stability and durability of YGHP were confirmed, affirming its potential for practical applications. Trapping experiments and electron spin resonance analyses identified active species generated by YGHP, namely •OH, •O2-, and h+, allowing for comprehensive analysis of the degradation mechanisms and pathways of AC. Overall, this investigation advances the development of efficient Z-scheme heterostructural materials and provides valuable insights into formulating sustainable remediation strategies for combatting AC contamination.
Keywords: GdYBiNbO7; Y2TmSbO7; Y2TmSbO7/GdYBiNbO7 heterojunction photocatalyst; acetochlor; degradation mechanism; degradation pathway; direct Z-scheme; photocatalytic activity; visible light exposure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures













Similar articles
-
Novel Visible Light-Driven Ho2InSbO7/Ag3PO4 Photocatalyst for Efficient Oxytetracycline Contaminant Degradation.Molecules. 2025 Aug 6;30(15):3289. doi: 10.3390/molecules30153289. Molecules. 2025. PMID: 40807463 Free PMC article.
-
The Fabrication and Property Characterization of a Ho2YSbO7/Bi2MoO6 Heterojunction Photocatalyst and the Application of the Photodegradation of Diuron under Visible Light Irradiation.Int J Mol Sci. 2024 Apr 17;25(8):4418. doi: 10.3390/ijms25084418. Int J Mol Sci. 2024. PMID: 38674003 Free PMC article.
-
Visible Light-Driven Direct Z-Scheme Ho2SmSbO7/YbDyBiNbO7 Heterojunction Photocatalyst for Efficient Degradation of Fenitrothion.Molecules. 2024 Dec 16;29(24):5930. doi: 10.3390/molecules29245930. Molecules. 2024. PMID: 39770019 Free PMC article.
-
Emerging bismuth stannate semiconductor and its photocatalytic applications in pollutant degradation via Z/S-scheme heterostructures.Environ Res. 2025 Aug 15;279(Pt 2):121670. doi: 10.1016/j.envres.2025.121670. Epub 2025 May 8. Environ Res. 2025. PMID: 40348258 Review.
-
Recent advances in photocatalytic removal of antiviral drugs by Z-scheme and S-scheme heterojunction.Environ Sci Pollut Res Int. 2024 Jun;31(28):40851-40872. doi: 10.1007/s11356-024-33876-9. Epub 2024 Jun 5. Environ Sci Pollut Res Int. 2024. PMID: 38837030 Review.
Cited by
-
Novel Visible Light-Driven Ho2InSbO7/Ag3PO4 Photocatalyst for Efficient Oxytetracycline Contaminant Degradation.Molecules. 2025 Aug 6;30(15):3289. doi: 10.3390/molecules30153289. Molecules. 2025. PMID: 40807463 Free PMC article.
References
-
- El-Saeid M.H., Alotaibi M.O., Alshabanat M., Al-Anazy M.M., Alharbi K.R., Altowyan A.S. Impact of Photolysis and TiO2 on Pesticides Degradation in Wastewater. Water. 2021;13:3080. doi: 10.3390/w13213080. - DOI
-
- Andronic L., Lelis M., Enesca A., Karazhanov S. Photocatalytic activity of defective black-titanium oxide photocatalysts towards pesticide degradation under UV/VIS irradiation. Surf. Interfaces. 2022;32:102123. doi: 10.1016/j.surfin.2022.102123. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

