LPA-Induced Thromboxane A2-Mediated Vasoconstriction Is Limited to Poly-Unsaturated Molecular Species in Mouse Aortas
- PMID: 38999980
- PMCID: PMC11241118
- DOI: 10.3390/ijms25136872
LPA-Induced Thromboxane A2-Mediated Vasoconstriction Is Limited to Poly-Unsaturated Molecular Species in Mouse Aortas
Abstract
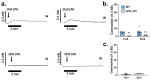
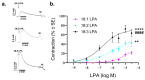
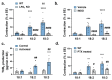
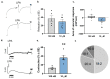
We have previously reported that, in aortic rings, 18:1 lysophosphatidic acid (LPA) can induce both vasodilation and vasoconstriction depending on the integrity of the endothelium. The predominant molecular species generated in blood serum are poly-unsaturated LPA species, yet the vascular effects of these species are largely unexplored. We aimed to compare the vasoactive effects of seven naturally occurring LPA species in order to elucidate their potential pathophysiological role in vasculopathies. Vascular tone was measured using myography, and thromboxane A2 (TXA2) release was detected by ELISA in C57Bl/6 mouse aortas. The Ca2+-responses to LPA-stimulated primary isolated endothelial cells were measured by Fluo-4 AM imaging. Our results indicate that saturated molecular species of LPA elicit no significant effect on the vascular tone of the aorta. In contrast, all 18 unsaturated carbon-containing (C18) LPAs (18:1, 18:2, 18:3) were effective, with 18:1 LPA being the most potent. However, following inhibition of cyclooxygenase (COX), these LPAs induced similar vasorelaxation, primarily indicating that the vasoconstrictor potency differed among these species. Indeed, C18 LPA evoked a similar Ca2+-signal in endothelial cells, whereas in endothelium-denuded aortas, the constrictor activity increased with the level of unsaturation, correlating with TXA2 release in intact aortas. COX inhibition abolished TXA2 release, and the C18 LPA induced vasoconstriction. In conclusion, polyunsaturated LPA have markedly increased TXA2-releasing and vasoconstrictor capacity, implying potential pathophysiological consequences in vasculopathies.
Keywords: lysophosphatidic acid; lysophosphatidic acid receptor 1; thromboxane; vasoconstriction.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
LPA1 receptor-mediated thromboxane A2 release is responsible for lysophosphatidic acid-induced vascular smooth muscle contraction.FASEB J. 2017 Apr;31(4):1547-1555. doi: 10.1096/fj.201600735R. Epub 2017 Jan 9. FASEB J. 2017. PMID: 28069828 Free PMC article.
-
Lysophosphatidic acid induces shear stress-dependent contraction in mouse aortic strip in situ.J Cardiovasc Pharmacol. 2013 Dec;62(6):530-8. doi: 10.1097/FJC.0000000000000013. J Cardiovasc Pharmacol. 2013. PMID: 24084212
-
Mechanisms underlying uridine adenosine tetraphosphate-induced vascular contraction in mouse aorta: Role of thromboxane and purinergic receptors.Vascul Pharmacol. 2015 Oct;73:78-85. doi: 10.1016/j.vph.2015.04.009. Epub 2015 Apr 25. Vascul Pharmacol. 2015. PMID: 25921923 Free PMC article.
-
Lysophosphatidic acid induces vasodilation mediated by LPA1 receptors, phospholipase C, and endothelial nitric oxide synthase.FASEB J. 2014 Feb;28(2):880-90. doi: 10.1096/fj.13-234997. Epub 2013 Nov 18. FASEB J. 2014. PMID: 24249637 Free PMC article.
-
Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries.Prostaglandins Other Lipid Mediat. 2018 Jan;134:32-37. doi: 10.1016/j.prostaglandins.2017.11.004. Epub 2017 Nov 24. Prostaglandins Other Lipid Mediat. 2018. PMID: 29180071 Review.
Cited by
-
Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis.J Clin Med. 2025 Mar 29;14(7):2352. doi: 10.3390/jcm14072352. J Clin Med. 2025. PMID: 40217801 Free PMC article. Review.
References
-
- Gonzalez-Gil I., Zian D., Vazquez-Villa H., Hernandez-Torres G., Martinez R.F., Khiar-Fernandez N., Rivera R., Kihara Y., Devesa I., Mathivanan S., et al. A Novel Agonist of the Type 1 Lysophosphatidic Acid Receptor (LPA(1)), UCM-05194, Shows Efficacy in Neuropathic Pain Amelioration. J. Med. Chem. 2020;63:2372–2390. doi: 10.1021/acs.jmedchem.9b01287. - DOI - PMC - PubMed
-
- Axelsson Raja A., Wakimoto H., DeLaughter D.M., Reichart D., Gorham J., Conner D.A., Lun M., Probst C.K., Sakai N., Knipe R.S., et al. Ablation of lysophosphatidic acid receptor 1 attenuates hypertrophic cardiomyopathy in a mouse model. Proc. Natl. Acad. Sci. USA. 2022;119:e2204174119. doi: 10.1073/pnas.2204174119. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

