Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9
- PMID: 38999987
- PMCID: PMC11240984
- DOI: 10.3390/ijms25136873
Functional and Structural Properties of Cytoplasmic Tropomyosin Isoforms Tpm1.8 and Tpm1.9
Abstract
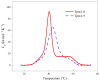
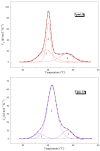
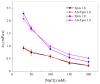
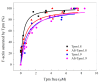
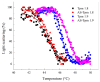
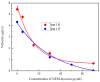
The actin cytoskeleton is one of the most important players in cell motility, adhesion, division, and functioning. The regulation of specific microfilament formation largely determines cellular functions. The main actin-binding protein in animal cells is tropomyosin (Tpm). The unique structural and functional diversity of microfilaments is achieved through the diversity of Tpm isoforms. In our work, we studied the properties of the cytoplasmic isoforms Tpm1.8 and Tpm1.9. The results showed that these isoforms are highly thermostable and differ in the stability of their central and C-terminal fragments. The properties of these isoforms were largely determined by the 6th exons. Thus, the strength of the end-to-end interactions, as well as the affinity of the Tpm molecule for F-actin, differed between the Tpm1.8 and Tpm1.9 isoforms. They were determined by whether an alternative internal exon, 6a or 6b, was included in the Tpm isoform structure. The strong interactions of the Tpm1.8 and Tpm1.9 isoforms with F-actin led to the formation of rigid actin filaments, the stiffness of which was measured using an optical trap. It is quite possible that the structural and functional features of the Tpm isoforms largely determine the appearance of these isoforms in the rigid actin structures of the cell cortex.
Keywords: actin cytoskeleton dynamics; actin filaments; actin-associated proteins; cytoplasmic isoforms of tropomyosin; differential scanning calorimetry; optical trap.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Khaitlina S.Y. International Review of Cell and Molecular Biology. Volume 318. Elsevier; Amsterdam, The Netherlands: 2015. Tropomyosin as a Regulator of Actin Dynamics; pp. 255–291. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

