Antibody-Drug Conjugates-Evolution and Perspectives
- PMID: 39000079
- PMCID: PMC11241239
- DOI: 10.3390/ijms25136969
Antibody-Drug Conjugates-Evolution and Perspectives
Abstract
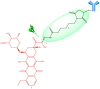
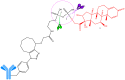
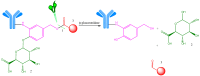
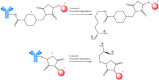
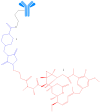
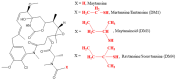
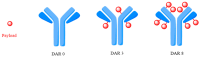
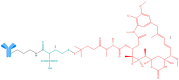
Antineoplastic therapy is one of the main research themes of this century. Modern approaches have been implemented to target and heighten the effect of cytostatic drugs on tumors and diminish their general/unspecific toxicity. In this context, antibody-drug conjugates (ADCs) represent a promising and successful strategy. The aim of this review was to assess different aspects regarding ADCs. They were presented from a chemical and a pharmacological perspective and aspects like structure, conjugation and development particularities alongside effects, clinical trials, safety issues and perspectives and challenges for future use of these drugs were discussed. Representative examples include but are not limited to the following main structural components of ADCs: monoclonal antibodies (trastuzumab, brentuximab), linkers (pH-sensitive, reduction-sensitive, peptide-based, phosphate-based, and others), and payloads (doxorubicin, emtansine, ravtansine, calicheamicin). Regarding pharmacotherapy success, the high effectiveness expectation associated with ADC treatment is supported by the large number of ongoing clinical trials. Major aspects such as development strategies are first discussed, advantages and disadvantages, safety and efficacy, offering a retrospective insight on the subject. The second part of the review is prospective, focusing on various plans to overcome the previously identified difficulties.
Keywords: antibody–drug conjugates; cancer; clinical trials; cytotoxic payload; efficacy; linker; monoclonal antibody; safety; targeted therapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Thurston D.E., Pysz I. Chemistry and Pharmacology of Anticancer Drugs. 2nd ed. CRC Press; Boca Raton, USA: 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

