Pills of Multi-Target H2S Donating Molecules for Complex Diseases
- PMID: 39000122
- PMCID: PMC11240940
- DOI: 10.3390/ijms25137014
Pills of Multi-Target H2S Donating Molecules for Complex Diseases
Abstract
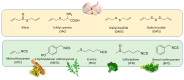
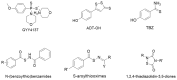
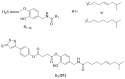
Among the various drug discovery methods, a very promising modern approach consists in designing multi-target-directed ligands (MTDLs) able to modulate multiple targets of interest, including the pathways where hydrogen sulfide (H2S) is involved. By incorporating an H2S donor moiety into a native drug, researchers have been able to simultaneously target multiple therapeutic pathways, resulting in improved treatment outcomes. This review gives the reader some pills of successful multi-target H2S-donating molecules as worthwhile tools to combat the multifactorial nature of complex disorders, such as inflammatory-based diseases and cancer, as well as cardiovascular, metabolic, and neurodegenerative disorders.
Keywords: H2S donors; hydrogen sulfide; molecular hybridization; multi-target compounds; multi-target directed ligand.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





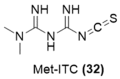


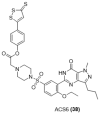
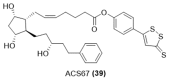

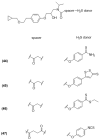
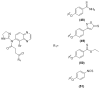




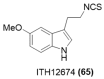

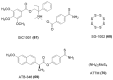
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

