The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation
- PMID: 39000151
- PMCID: PMC11241625
- DOI: 10.3390/ijms25137047
The Impact of Virgin and Aged Microstructured Plastics on Proteins: The Case of Hemoglobin Adsorption and Oxygenation
Abstract
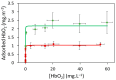
Plastic particles, particularly micro- and nanoparticles, are emerging pollutants due to the ever-growing amount of plastics produced across a wide variety of sectors. When plastic particles enter a biological medium, they become surrounded by a corona, giving them their biological identity and determining their interactions in the living environment and their biological effects. Here, we studied the interactions of microstructured plastics with hemoglobin (Hb). Virgin polyethylene microparticles (PEMPs) and polypropylene microparticles (PPMPs) as well as heat- or irradiation-aged microparticles (ag-PEMPs and ag-PPMPs) were used to quantify Hb adsorption. Polypropylene filters (PP-filters) were used to measure the oxygenation of adsorbed Hb. Microstructured plastics were characterized using optical microscopy, SAXS, ATR-FTIR, XPS, and Raman spectroscopy. Adsorption isotherms showed that the Hb corona thickness is larger on PPMPs than on PEMPs and Hb has a higher affinity for PPMPs than for PEMPs. Hb had a lower affinity for ag-PEMPs and ag-PPMPs, but they can be adsorbed in larger amounts. The presence of partial charges on the plastic surface and the oxidation rate of microplastics may explain these differences. Tonometry experiments using an original method, the diffuse reflection of light, showed that adsorbed Hb on PP-filters retains its cooperativity, but its affinity for O2 decreases significantly.
Keywords: hemoglobin; hemoglobin activity; hemoglobin adsorption; microstructured plastics; oxygenation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Wypych G. Handbook of Polymers. 3rd ed. ChemTec Publishing; Toronto, ON, Canada: 2022.
-
- Zweifel H., Maier R., Schiller M. Plastics Additives Handbook. 6th ed. Hanser Publications; Munich, Germany: 2009.
-
- Pfaendner R. How will additives shape the future of plastics? Polym. Degrad. Stab. 2006;91:2249–2256. doi: 10.1016/j.polymdegradstab.2005.10.017. - DOI
-
- Alegado J.E., Aliño J.M., Chow S.C.F., Diola M.B.L., Jagath P., Gamaralalage D., Gurrero L., Hengesbaugh M., Hernandez V., Ishimura Y., et al. In: Plastic Atlas Asia Edition. 1st ed. Li K., Kunze C., editors. Heinrich Boell Stiftung, Break Free from Pacific Asia Pacific, Institute for Global Environmental Strategies Publishers; Hong Kong: 2021.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

