Mitigating the Functional Deficit after Neurotoxic Motoneuronal Loss by an Inhibitor of Mitochondrial Fission
- PMID: 39000168
- PMCID: PMC11241433
- DOI: 10.3390/ijms25137059
Mitigating the Functional Deficit after Neurotoxic Motoneuronal Loss by an Inhibitor of Mitochondrial Fission
Abstract
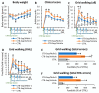
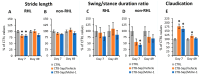
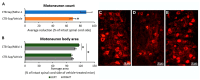
Amyotrophic lateral sclerosis (ALS) is an extremely complex neurodegenerative disease involving different cell types, but motoneuronal loss represents its main pathological feature. Moreover, compensatory plastic changes taking place in parallel to neurodegeneration are likely to affect the timing of ALS onset and progression and, interestingly, they might represent a promising target for disease-modifying treatments. Therefore, a simplified animal model mimicking motoneuronal loss without the other pathological aspects of ALS has been established by means of intramuscular injection of cholera toxin-B saporin (CTB-Sap), which is a targeted neurotoxin able to kill motoneurons by retrograde suicide transport. Previous studies employing the mouse CTB-Sap model have proven that spontaneous motor recovery is possible after a subtotal removal of a spinal motoneuronal pool. Although these kinds of plastic changes are not enough to counteract the functional effects of the progressive motoneuron degeneration, it would nevertheless represent a promising target for treatments aiming to postpone ALS onset and/or delay disease progression. Herein, the mouse CTB-Sap model has been used to test the efficacy of mitochondrial division inhibitor 1 (Mdivi-1) as a tool to counteract the CTB-Sap toxicity and/or to promote neuroplasticity. The homeostasis of mitochondrial fission/fusion dynamics is indeed important for cell integrity, and it could be affected during neurodegeneration. Lesioned mice were treated with Mdivi-1 and then examined by a series of behavioral test and histological analyses. The results have shown that the drug may be capable of reducing functional deficits after the lesion and promoting synaptic plasticity and neuroprotection, thus representing a putative translational approach for motoneuron disorders.
Keywords: Mdivi-1; behavioral test; cholera toxin-B saporin; gastrocnemius muscle; mouse; neurodegeneration; spinal cord; synaptic plasticity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

