GBA1-Associated Parkinson's Disease Is a Distinct Entity
- PMID: 39000225
- PMCID: PMC11241486
- DOI: 10.3390/ijms25137102
GBA1-Associated Parkinson's Disease Is a Distinct Entity
Abstract
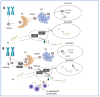
GBA1-associated Parkinson's disease (GBA1-PD) is increasingly recognized as a distinct entity within the spectrum of parkinsonian disorders. This review explores the unique pathophysiological features, clinical progression, and genetic underpinnings that differentiate GBA1-PD from idiopathic Parkinson's disease (iPD). GBA1-PD typically presents with earlier onset and more rapid progression, with a poor response to standard PD medications. It is marked by pronounced cognitive impairment and a higher burden of non-motor symptoms compared to iPD. Additionally, patients with GBA1-PD often exhibit a broader distribution of Lewy bodies within the brain, accentuating neurodegenerative processes. The pathogenesis of GBA1-PD is closely associated with mutations in the GBA1 gene, which encodes the lysosomal enzyme beta-glucocerebrosidase (GCase). In this review, we discuss two mechanisms by which GBA1 mutations contribute to disease development: 'haploinsufficiency,' where a single functional gene copy fails to produce a sufficient amount of GCase, and 'gain of function,' where the mutated GCase acquires harmful properties that directly impact cellular mechanisms for alpha-synuclein degradation, leading to alpha-synuclein aggregation and neuronal cell damage. Continued research is advancing our understanding of how these mechanisms contribute to the development and progression of GBA1-PD, with the 'gain of function' mechanism appearing to be the most plausible. This review also explores the implications of GBA1 mutations for therapeutic strategies, highlighting the need for early diagnosis and targeted interventions. Currently, small molecular chaperones have shown the most promising clinical results compared to other agents. This synthesis of clinical, pathological, and molecular aspects underscores the assertion that GBA1-PD is a distinct clinical and pathobiological PD phenotype, necessitating specific management and research approaches to better understand and treat this debilitating condition.
Keywords: GBA1 variants; GBA1-associated Parkinson disease; Parkinson’s disease; clinical presentation and course; genotype-phenotype correlations; pathophysiology and molecular mechanisms; treatment options.
Conflict of interest statement
M.H.: Honorarium from Agyany Pharma/Israel and member of Agyany Pharma; M.J.I., A.R. and A.Z. are employees of Agyany Pharma; V.S. and J.L. are working as consultants for Agyany Pharma; S.H.-B. is member of the SAB of Agyany Pharma; S.R.-V. is Principle Investigator of a clinical study being conducted by Agyany Pharma; P.S.: Honorarium from AbbVie, Lundbeck, SAB of Agyany; Authors A.S. and A.R. are employees of Rare Disease Consulting RCV GmbH; the remaining authors declare no conflicts of interest.
Figures
Similar articles
-
Targeting the GBA1 pathway to slow Parkinson disease: Insights into clinical aspects, pathogenic mechanisms and new therapeutic avenues.Pharmacol Ther. 2023 Jun;246:108419. doi: 10.1016/j.pharmthera.2023.108419. Epub 2023 Apr 19. Pharmacol Ther. 2023. PMID: 37080432 Review.
-
The Molecular Impact of Glucosylceramidase Beta 1 (Gba1) in Parkinson's Disease: a New Genetic State of the Art.Mol Neurobiol. 2024 Sep;61(9):6754-6770. doi: 10.1007/s12035-024-04008-8. Epub 2024 Feb 13. Mol Neurobiol. 2024. PMID: 38347286 Review.
-
Lysosomal functions and dysfunctions: Molecular and cellular mechanisms underlying Gaucher disease and its association with Parkinson disease.Adv Drug Deliv Rev. 2022 Aug;187:114402. doi: 10.1016/j.addr.2022.114402. Epub 2022 Jun 25. Adv Drug Deliv Rev. 2022. PMID: 35764179 Review.
-
The relationship between glucocerebrosidase mutations and Parkinson disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):77-90. doi: 10.1111/jnc.13385. Epub 2016 Feb 10. J Neurochem. 2016. PMID: 26860875 Free PMC article. Review.
-
AAV delivery of GBA1 suppresses α-synuclein accumulation in Parkinson's disease models and restores functions in Gaucher's disease models.PLoS One. 2025 May 7;20(5):e0321145. doi: 10.1371/journal.pone.0321145. eCollection 2025. PLoS One. 2025. PMID: 40333681 Free PMC article.
Cited by
-
CoPPIs algorithm: a tool to unravel protein cooperative strategies in pathophysiological conditions.Brief Bioinform. 2025 Mar 4;26(2):bbaf146. doi: 10.1093/bib/bbaf146. Brief Bioinform. 2025. PMID: 40194557 Free PMC article.
-
Targeting Glucose Metabolism: A Novel Therapeutic Approach for Parkinson's Disease.Cells. 2024 Nov 13;13(22):1876. doi: 10.3390/cells13221876. Cells. 2024. PMID: 39594624 Free PMC article. Review.
-
Prodromal Parkinsonian Features in Carriers of Gaucher Disease Compared to Controls.Life (Basel). 2025 Jun 13;15(6):952. doi: 10.3390/life15060952. Life (Basel). 2025. PMID: 40566604 Free PMC article.
-
Sidransky Syndrome-GBA1-Related Parkinson's Disease and Its Targeted Therapies.Int J Mol Sci. 2025 Apr 6;26(7):3435. doi: 10.3390/ijms26073435. Int J Mol Sci. 2025. PMID: 40244386 Free PMC article. Review.
-
Olfactory Perception in Parkinson's Disease: The Impact of GBA1 Variants (Sidransky Syndrome).Int J Mol Sci. 2025 May 30;26(11):5258. doi: 10.3390/ijms26115258. Int J Mol Sci. 2025. PMID: 40508068 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

