Regulatory T Cell Dysfunction in Autoimmune Diseases
- PMID: 39000278
- PMCID: PMC11241405
- DOI: 10.3390/ijms25137171
Regulatory T Cell Dysfunction in Autoimmune Diseases
Abstract
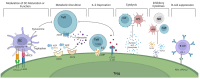
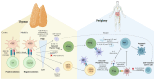
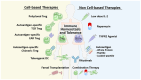
Regulatory T cells (Tregs), a suppressive subpopulation of T cells, are potent mediators of peripheral tolerance, responsible for immune homeostasis. Many autoimmune diseases exhibit disruptions in Treg function or quantity, resulting in an imbalance between protective and pathogenic immune cells. Selective expansion or manipulation of Tregs is a promising therapeutic approach for autoimmune diseases. However, the extensive diversity of Treg subpopulations and the multiple approaches used for Treg identification leads to high complexity, making it difficult to develop a successful treatment capable of modulating Tregs. In this review, we describe the suppressive mechanisms, subpopulations, classification, and identification methodology for Tregs, and their role in the pathogenesis of autoimmune diseases.
Keywords: autoimmune disease; immunology; regulatory T cell.
Conflict of interest statement
Tiago R. Matos is an employee of Sanofi and may hold shares and/or stock options in the company.
Figures
References
-
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. - DOI - PubMed
-
- Wildin R.S., Ramsdell F., Peake J., Faravelli F., Casanova J.L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. - DOI - PubMed
-
- Goudy K., Aydin D., Barzaghi F., Gambineri E., Vignoli M., Ciullini Mannurita S., Doglioni C., Ponzoni M., Cicalese M.P., Assanelli A., et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin. Immunol. 2013;146:248–261. doi: 10.1016/j.clim.2013.01.004. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

