CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies
- PMID: 39000281
- PMCID: PMC11241811
- DOI: 10.3390/ijms25137174
CAR-T Cells Therapy in Glioblastoma: A Systematic Review on Molecular Targets and Treatment Strategies
Abstract
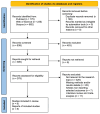
The most common primary brain tumor is glioblastoma (GBM), yet the current therapeutic options for this disease are not promising. Although immunotherapeutic techniques have shown poor success in GBM thus far despite efforts, new developments provide optimism. One of these developments is chimeric antigen receptor (CAR)-T cell treatment, which includes removing and genetically modifying autologous T cells to produce a receptor that targets a GBM antigen before reintroducing the cells into the patient's body. A number of preclinical studies have produced encouraging results, which have led to the start of clinical trials assessing these CAR-T cell treatments for GBM and other brain tumors. Although results in tumors such as diffuse intrinsic pontine gliomas and lymphomas have been promising, preliminary findings in GBM have not produced any clinical benefits. The paucity of particular antigens in GBM, their inconsistent expression patterns, and the possible immunoediting-induced loss of these antigens after antigen-targeted therapy are some possible causes for this discrepancy. The goal of this systematic literature review is to assess potential approaches for creating CAR-T cells that are more effective for this indication, as well as the clinical experiences that are already being had with CAR-T cell therapy in GBM. Up until 9 May 2024, a thorough search was carried out across the three main medical databases: PubMed, Web of Science, and Scopus. Relevant Medical Subject Heading (MeSH) terms and keywords associated with "glioblastoma", "CAR-T", "T cell therapy", "overall survival", and "progression free survival" were employed in the search approach. Preclinical and clinical research on the application of CAR-T cells as a therapeutic approach for GBM are included in the review. A total of 838 papers were identified. Of these, 379 articles were assessed for eligibility, resulting in 8 articles meeting the inclusion criteria. The included studies were conducted between 2015 and 2023, with a total of 151 patients enrolled. The studies varied in CAR-T cell types. EGFRvIII CAR-T cells were the most frequently investigated, used in three studies (37.5%). Intravenous delivery was the most common method of delivery (62.5%). Median OS ranged from 5.5 to 11.1 months across the studies. PFS was reported in only two studies, with values of 7.5 months and 1.3 months. This systematic review highlights the evolving research on CAR-T cell therapy for GBM, emphasizing its potential despite challenges. Targeting antigens like EGFRvIII and IL13Rα2 shows promise in treating recurrent GBM. However, issues such as antigen escape, tumor heterogeneity, and immunosuppression require further optimization. Innovative delivery methods, combination therapies, and personalized approaches are crucial for enhancing CAR-T cell efficacy. Ongoing research is essential to refine these therapies and improve outcomes for GBM patients.
Keywords: CAR T cell; glioblastoma; immunotherapy; molecular targets; systematic reviews.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Vik-Mo E.O., Nyakas M., Mikkelsen B.V., Moe M.C., Due-Tønnesen P., Suso E.M.I., Sæbøe-Larssen S., Sandberg C., Brinchmann J.E., Helseth E., et al. Therapeutic Vaccination against Autologous Cancer Stem Cells with mRNA-Transfected Dendritic Cells in Patients with Glioblastoma. Cancer Immunol. Immunother. 2013;62:1499–1509. doi: 10.1007/s00262-013-1453-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

