Pirfenidone Prevents Heart Fibrosis during Chronic Chagas Disease Cardiomyopathy
- PMID: 39000409
- PMCID: PMC11242150
- DOI: 10.3390/ijms25137302
Pirfenidone Prevents Heart Fibrosis during Chronic Chagas Disease Cardiomyopathy
Abstract
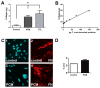
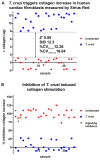
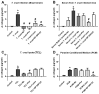
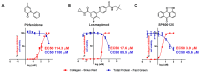
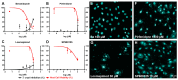
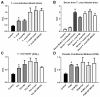
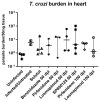
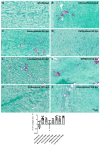
Cardiac fibrosis is a severe outcome of Chagas disease (CD), caused by the protozoan Trypanosoma cruzi. Clinical evidence revealed a correlation between fibrosis levels with impaired cardiac performance in CD patients. Therefore, we sought to analyze the effect of inhibitors of TGF-β (pirfenidone), p38-MAPK (losmapimod) and c-Jun (SP600125) on the modulation of collagen deposition in cardiac fibroblasts (CF) and in vivo models of T. cruzi chronic infection. Sirius Red/Fast Green dye was used to quantify both collagen expression and total protein amount, assessing cytotoxicity. The compounds were also used to treat C57/Bl6 mice chronically infected with T. cruzi, Brazil strain. We identified an anti-fibrotic effect in vitro for pirfenidone (TGF-β inhibitor, IC50 114.3 μM), losmapimod (p38 inhibitor, IC50 17.6 μM) and SP600125 (c-Jun inhibitor, IC50 3.9 μM). This effect was independent of CF proliferation since these compounds do not affect T. cruzi-induced host cell multiplication as measured by BrdU incorporation. Assays of chronic infection of mice with T. cruzi have shown a reduction in heart collagen by pirfenidone. These results propose a novel approach to fibrosis therapy in CD, with the prospect of repurposing pirfenidone to prevent the onset of ECM accumulation in the hearts of the patients.
Keywords: Chagas disease; heart fibrosis; pirfenidone.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the study’s design; in the collection, analysis, or interpretation of data; in the manuscript’s writing; or in the decision to publish the results.
Figures

References
-
- World Health Organization Chagas Disease (Also Known as American Trypanosomiasis) [(accessed on 13 November 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(america...
-
- Chagas C. Nova Tripanozomiaze Humana. Mem. Inst. Oswaldo Cruz. 1909;1:74–276. doi: 10.1590/S0074-02761909000200008. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

