Structural Studies of the Taurine Transporter: A Potential Biological Target from the GABA Transporter Subfamily in Cancer Therapy
- PMID: 39000444
- PMCID: PMC11242302
- DOI: 10.3390/ijms25137339
Structural Studies of the Taurine Transporter: A Potential Biological Target from the GABA Transporter Subfamily in Cancer Therapy
Abstract
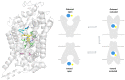
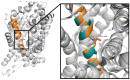
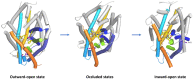
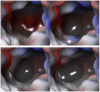
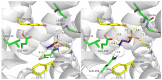
The taurine transporter (TauT, SLC6A6) is a member of the solute carrier 6 (SLC6) family, which plays multiple physiological roles. The SLC6 family is divided into four subfamilies: GABA (γ-aminobutyric acid), monoamine, glycine and neutral amino acid transporters. Proteins from the GABA group, including the taurine transporter, are primarily considered therapeutic targets for treating central nervous system disorders. However, recent studies have suggested that inhibitors of SLC6A6 could also serve as anticancer agents. Overexpression of TauT has been associated with the progression of colon and gastric cancer. The pool of known ligands of this transporter is limited and the exact spatial structure of taurine transporter remains unsolved. Understanding its structure could aid in the development of novel inhibitors. Therefore, we utilized homology modelling techniques to create models of TauT. Docking studies and molecular dynamics simulations were conducted to describe protein-ligand interactions. We compared the obtained information for TauT with literature data on other members of the GABA transporter group. Our in silico analysis allowed us to characterize the transporter structure and point out amino acids crucial for ligand binding: Glu406, Gly62 and Tyr138. The significance of selected residues was confirmed through structural studies of mutants. These results will aid in the development of novel taurine transporter inhibitors, which can be explored as anticancer agents.
Keywords: SLC6A6; cancer; docking; homology modelling; molecular dynamics; structure; taurine transporter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Saks V., Kaambre T., Guzun R., Anmann T., Sikk P., Schlattner U., Wallimann T., Aliev M., Vendelin M. Creatine and Creatine Kinase in Health and Disease. [(accessed on 18 May 2021)];Subcell. Biochem. 2007 46:27–65. Available online: http://www.researchgate.net/publication/240997256_The_Creatine_Kinase_Ph.... - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

