Toxic Advanced Glycation End-Products Inhibit Axonal Elongation Mediated by β-Tubulin Aggregation in Mice Optic Nerves
- PMID: 39000515
- PMCID: PMC11242247
- DOI: 10.3390/ijms25137409
Toxic Advanced Glycation End-Products Inhibit Axonal Elongation Mediated by β-Tubulin Aggregation in Mice Optic Nerves
Abstract
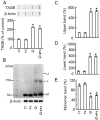
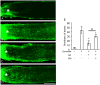
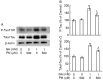
Advanced glycation end-products (AGEs) form through non-enzymatic glycation of various proteins. Optic nerve degeneration is a frequent complication of diabetes, and retinal AGE accumulation is strongly linked to the development of diabetic retinopathy. Type 2 diabetes mellitus is a major risk factor for Alzheimer's disease (AD), with patients often exhibiting optic axon degeneration in the nerve fiber layer. Notably, a gap exists in our understanding of how AGEs contribute to neuronal degeneration in the optic nerve within the context of both diabetes and AD. Our previous work demonstrated that glyceraldehyde (GA)-derived toxic advanced glycation end-products (TAGE) disrupt neurite outgrowth through TAGE-β-tubulin aggregation and tau phosphorylation in neural cultures. In this study, we further illustrated GA-induced suppression of optic nerve axonal elongation via abnormal β-tubulin aggregation in mouse retinas. Elucidating this optic nerve degeneration mechanism holds promise for bridging the knowledge gap regarding vision loss associated with diabetes mellitus and AD.
Keywords: axonal elongation; glyceraldehyde; tau; toxic advanced glycation end-products; β-tubulin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Yan S.D., Yan S.F., Chen X., Fu J., Chen M., Kuppusamy P., Smith M.A., Perry G., Godman G.C., Nawroth P., et al. Non-enzymatically glycated tau in Alzheimer’s disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat. Med. 1995;1:693–699. doi: 10.1038/nm0795-693. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

