Multi-Wavelength Raman Differentiation of Malignant Skin Neoplasms
- PMID: 39000528
- PMCID: PMC11242141
- DOI: 10.3390/ijms25137422
Multi-Wavelength Raman Differentiation of Malignant Skin Neoplasms
Abstract
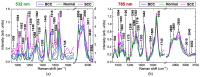
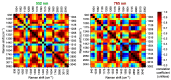
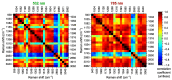
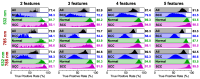
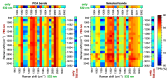
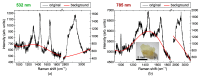
Raman microspectroscopy has become an effective method for analyzing the molecular appearance of biomarkers in skin tissue. For the first time, we acquired in vitro Raman spectra of healthy and malignant skin tissues, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), at 532 and 785 nm laser excitation wavelengths in the wavenumber ranges of 900-1800 cm-1 and 2800-3100 cm-1 and analyzed them to find spectral features for differentiation between the three classes of the samples. The intensity ratios of the bands at 1268, 1336, and 1445 cm-1 appeared to be the most reliable criteria for the three-class differentiation at 532 nm excitation, whereas the bands from the higher wavenumber region (2850, 2880, and 2930 cm-1) were a robust measure of the increased protein/lipid ratio in the tumors at both excitation wavelengths. Selecting ratios of the three bands from the merged (532 + 785) dataset made it possible to increase the accuracy to 87% for the three classes and reach the specificities for BCC + SCC equal to 87% and 81% for the sensitivities of 95% and 99%, respectively. Development of multi-wavelength excitation Raman spectroscopic techniques provides a versatile non-invasive tool for research of the processes in malignant skin tumors, as well as other forms of cancer.
Keywords: Raman microspectroscopy; basal cell carcinoma; multispectral analysis; squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rimskaya E., Shelygina S., Timurzieva A., Saraeva I., Perevedentseva E., Melnik N., Kudrin K., Reshetov D., Kudryashov S. Multispectral Raman Differentiation of Malignant Skin Neoplasms In Vitro: Search for Specific Biomarkers and Optimal Wavelengths. Int. J. Mol. Sci. 2023;24:14748. doi: 10.3390/ijms241914748. - DOI - PMC - PubMed
-
- Goel A., Tsikritsis D., Belsey N.A., Pendlington R., Glavin S., Chen T. Measurement of chemical penetration in skin using Stimulated Raman scattering microscopy and multivariate curve resolution-alternating least squares. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023;296:122639. doi: 10.1016/j.saa.2023.122639. - DOI - PubMed
-
- Gniadecka M., Philipsen P.A., Wessel S., Gniadecki R., Wulf H.C., Sigurdsson S., Nielsen O.F., Christensen D.H., Hercogova J., Rossen K., et al. Melanoma Diagnosis by Raman Spectroscopy and Neural Networks: Structure Alterations in Proteins and Lipids in Intact Cancer Tissue. J. Investig. Dermatol. 2004;122:443–449. doi: 10.1046/j.0022-202X.2004.22208.x. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

