Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals
- PMID: 39000669
- PMCID: PMC11244320
- DOI: 10.3390/polym16131815
Microspheres Based on Blends of Chitosan Derivatives with Carrageenan as Vitamin Carriers in Cosmeceuticals
Abstract
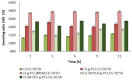
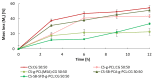
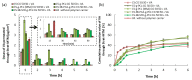
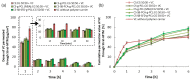
Chitosan (CS) has a natural origin and is a biodegradable and biocompatible polymer with many skin-beneficial properties successfully used in the cosmetics and pharmaceutical industry. CS derivatives, especially those synthesized via a Schiff base reaction, are very important due to their unique antimicrobial activity. This study demonstrates research results on the use of hydrogel microspheres made of [chitosan-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan)], [chitosan-2-pyridinecarboxaldehyde-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan), and chitosan-sodium-4-formylbenzene-1,3-disulfonate-graft-poly(ε-caprolactone)]-blend-(ĸ-carrageenan) as innovative vitamin carriers for cosmetic formulation. A permeation study of retinol (vitamin A), L-ascorbic acid (vitamin C), and α-tocopherol (vitamin E) from the cream through a human skin model by the Franz Cell measurement system was presented. The quantitative analysis of the release of the vitamins added to the cream base, through the membrane, imitating human skin, showed a promising profile of its release/penetration, which is promising for the development of a cream with anti-aging properties. Additionally, the antibacterial activity of the polymers from which the microspheres are made allows for the elimination of preservatives and parabens as cosmetic formulation ingredients.
Keywords: chitosan Schiff base; controlled release; cosmetic formulations; microspheres; trans-epidermal absorption; vitamin; κ-carrageenan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Bactericidal Chitosan Derivatives and Their Superabsorbent Blends with ĸ-Carrageenan.Int J Mol Sci. 2024 Apr 20;25(8):4534. doi: 10.3390/ijms25084534. Int J Mol Sci. 2024. PMID: 38674119 Free PMC article.
-
[Preparation of kappa-carrageenan-chitosan polyelectrolyte gel beads].Zhongguo Zhong Yao Za Zhi. 2012 Feb;37(4):466-70. Zhongguo Zhong Yao Za Zhi. 2012. PMID: 22667145 Chinese.
-
Electrospun poly(ω-pentadecalactone-co-ε-caprolactone)/gelatin/chitosan ternary nanofibers with antibacterial activity for treatment of skin infections.Eur J Pharm Sci. 2022 Mar 1;170:106113. doi: 10.1016/j.ejps.2021.106113. Epub 2022 Jan 2. Eur J Pharm Sci. 2022. PMID: 34986416
-
Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics.Int J Toxicol. 2005;24 Suppl 2:51-111. doi: 10.1080/10915810590953851. Int J Toxicol. 2005. PMID: 16154915 Review.
-
Implication of parabens in cosmetics and cosmeceuticals: Advantages and limitations.J Cosmet Dermatol. 2022 Aug;21(8):3265-3271. doi: 10.1111/jocd.14775. Epub 2022 Jan 23. J Cosmet Dermatol. 2022. PMID: 35032353 Review.
Cited by
-
Box-Behnken Design Assisted Optimization and Characterization of Chitosan Film for Simultaneous Topical Delivery of Ascorbic Acid and Metronidazole.Pharmaceutics. 2025 Apr 24;17(5):562. doi: 10.3390/pharmaceutics17050562. Pharmaceutics. 2025. PMID: 40430855 Free PMC article.
References
-
- Sikorska W., Musioł M., Rydz J., Zięba M., Rychter P., Lewicka K., Šiškova A., Mosnáčková K., Kowalczuk M., Adamus G. Prediction Studies of Environment-Friendly Biodegradable Polymeric Packaging Based on PLA. Influence of Specimens’ Thickness on the Hydrolytic Degradation Profile. Waste Manag. 2018;78:938–947. doi: 10.1016/j.wasman.2018.07.014. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

