Determination of Partial Propagation Velocity and Partial Isentropic Compressibility Coefficient in Water-Ethanol System
- PMID: 39000840
- PMCID: PMC11244284
- DOI: 10.3390/s24134061
Determination of Partial Propagation Velocity and Partial Isentropic Compressibility Coefficient in Water-Ethanol System
Abstract
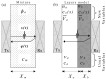
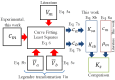
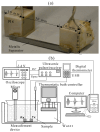
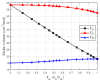
This study introduces an innovative approach to the layered model, emphasizing the physical-chemical characterization of miscible liquid systems through ultrasonic techniques, with a specific focus on the water-ethanol system used in pharmaceutical formulations. Traditional characterization methods, while effective, face challenges due to the complex nature of solutions, such as the need for large pressure variations and strict temperature control. The proposed approach integrates partial molar volumes and partial propagation velocity functions into the layered model, enabling a nuanced understanding of miscibility and interactions. Ultrasonic techniques are used to calculate the isentropic compressibility coefficient for each component of the mixture as well as the total value using an additive mixing rule. Unlike conventional methods, this technique uses tabulated and experimental data to estimate the propagation velocity in the mixture, leading to a more precise computation of the isentropic compressibility coefficient. The results indicate a significant improvement in predicting the behavior of the water-ethanol system compared to the classical layered model. The methodology demonstrates the potential to provide new physicochemical insights that can be applied to other miscible systems beyond water-ethanol. This research has implications for improving the efficiency and accuracy of liquid medication formulations in the pharmaceutical industry.
Keywords: layered models; partial properties; propagation velocity; water–ethanol.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Jiménez J., Manrique J., Martínez F. Effect of temperature on some volumetric properties for ethanol+ water mixtures. Rev. Colomb. Cienc. Quím Farm. 2004;33:145–155.
-
- Hoche S., Hussein M.A., Becker T. Critical process parameter of alcoholic yeast fermentation: Speed of sound and density in the temperature range 5–30 °C. Int. J. Food Sci. Technol. 2014;49:2441–2448. doi: 10.1111/ijfs.12566. - DOI
-
- Figueiredo M.K.K., Costa-Felix R.P., Maggi L.E., Alvarenga A.V., Romeiro G.A. Biofuel ethanol adulteration detection using an ultrasonic measurement method. Fuel. 2012;91:209–212. doi: 10.1016/j.fuel.2011.08.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

