External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study
- PMID: 39001284
- PMCID: PMC11241557
- DOI: 10.3390/diagnostics14131394
External Validation of the Machine Learning-Based Thermographic Indices for Rheumatoid Arthritis: A Prospective Longitudinal Study
Abstract
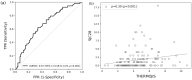
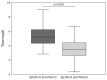
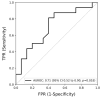
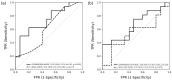
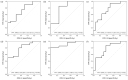
External validation is crucial in developing reliable machine learning models. This study aimed to validate three novel indices-Thermographic Joint Inflammation Score (ThermoJIS), Thermographic Disease Activity Index (ThermoDAI), and Thermographic Disease Activity Index-C-reactive protein (ThermoDAI-CRP)-based on hand thermography and machine learning to assess joint inflammation and disease activity in rheumatoid arthritis (RA) patients. A 12-week prospective observational study was conducted with 77 RA patients recruited from rheumatology departments of three hospitals. During routine care visits, indices were obtained at baseline and week 12 visits using a pre-trained machine learning model. The performance of these indices was assessed cross-sectionally and longitudinally using correlation coefficients, the area under the receiver operating curve (AUROC), sensitivity, specificity, and positive and negative predictive values. ThermoDAI and ThermoDAI-CRP correlated with CDAI, SDAI, and DAS28-CRP cross-sectionally (ρ = 0.81; ρ = 0.83; ρ = 0.78) and longitudinally (ρ = 0.55; ρ = 0.61; ρ = 0.60), all p < 0.001. ThermoDAI and ThermoDAI-CRP also outperformed Patient Global Assessment (PGA) and PGA + C-reactive protein (CRP) in detecting changes in 28-swollen joint counts (SJC28). ThermoJIS had an AUROC of 0.67 (95% CI, 0.58 to 0.76) for detecting patients with swollen joints and effectively identified patients transitioning from SJC28 > 1 at baseline visit to SJC28 ≤ 1 at week 12 visit. These results support the effectiveness of ThermoJIS in assessing joint inflammation, as well as ThermoDAI and ThermoDAI-CRP in evaluating disease activity in RA patients.
Keywords: artificial intelligence; external validation; machine learning; rheumatoid arthritis; thermography.
Conflict of interest statement
I.M.-I. and M.A.M.-L. are shareholders in Singularity Biomed. P.F., D.O., P.M.-O., and C.B. are employees of Eli Lilly and Company. The other authors declare no conflicts of interest regarding this work.
Figures


References
-
- Grigor C., Capell H., Stirling A., McMahon A.D., Lock P., Vallance R., Kincaid W., Porter D. Effect of a Treatment Strategy of Tight Control for Rheumatoid Arthritis (the TICORA Study): A Single-Blind Randomised Controlled Trial. Lancet. 2004;364:263–269. doi: 10.1016/S0140-6736(04)16676-2. - DOI - PubMed
-
- Verstappen S.M.M., Jacobs J.W.G., van der Veen M.J., Heurkens A.H.M., Schenk Y., ter Borg E.J., Blaauw A.a.M., Bijlsma J.W.J. Utrecht Rheumatoid Arthritis Cohort study group Intensive Treatment with Methotrexate in Early Rheumatoid Arthritis: Aiming for Remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an Open-Label Strategy Trial) Ann. Rheum. Dis. 2007;66:1443–1449. doi: 10.1136/ard.2007.071092. - DOI - PMC - PubMed
-
- Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A., McInnes I.B., Sepriano A., van Vollenhoven R.F., de Wit M., et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann. Rheum. Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

