Evolving Precision First-Line Systemic Treatment for Patients with Unresectable Non-Small Cell Lung Cancer
- PMID: 39001412
- PMCID: PMC11240640
- DOI: 10.3390/cancers16132350
Evolving Precision First-Line Systemic Treatment for Patients with Unresectable Non-Small Cell Lung Cancer
Abstract
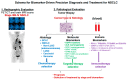
First-line systemic therapy for patients with advanced or metastatic non-small cell lung cancer (NSCLC) has rapidly evolved over the past two decades. First, molecularly targeted therapy for a growing number of gain-of-function molecular targets has been shown to improve progression-free survival (PFS) and overall survival (OS) with favorable toxicity profiles compared to platinum-containing chemotherapy and can be given as first-line systemic therapy in ~25% of patients with NSCLC. Actionable genetic alterations include EGFR, BRAF V600E, and MET exon 14 splicing site-sensitizing mutations, as well as ALK-, ROS1-, RET-, and NTRK-gene fusions. Secondly, inhibitors of programmed cell death protein 1 or its ligand 1 (PD-1/L1) such as pembrolizumab, atezolizumab, or cemiplimab monotherapy have become a standard of care for ~25% of patients with NSCLC whose tumors have high PD-L1 expression (total proportion score (TPS) ≥50%) and no sensitizing EGFR/ALK alterations. Lastly, for the remaining ~50% of patients who are fit and whose tumors have no or low PD-L1 expression (TPS of 0-49%) and no sensitizing EGFR/ALK aberrations, platinum-containing chemotherapy with the addition of a PD-1/L1 inhibitor alone or in combination of a cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor improves PFS and OS compared to chemotherapy alone. The objectives of this review are to summarize the current data and perspectives on first-line systemic treatment in patients with unresectable NSCLC and propose a practical algorithm for implementing precision biomarker testing at diagnosis.
Keywords: CTLA-4; NSCLC; PD-1; PD-L1; biomarkers; first-line therapy; immune biomarkers; immune checkpoint inhibitors; molecular targets.
Conflict of interest statement
T.L. receives research grants to conduct clinical trials as principal investigator at local institute from AbbVie Inc., Astellas, AstraZeneca, BioNTech, Chugai Pharma, Duality Biologics, Genentech/LaRoche, Jounce Therapeutics, LabyRx Immuno-Oncology, Merck, OncoC4, Novartis, RasCal Therapeutics, Tempus, and Xilio Therapeutics.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

