The Immunotherapy of Acute Myeloid Leukemia: A Clinical Point of View
- PMID: 39001421
- PMCID: PMC11240611
- DOI: 10.3390/cancers16132359
The Immunotherapy of Acute Myeloid Leukemia: A Clinical Point of View
Abstract
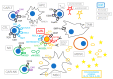
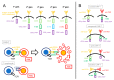
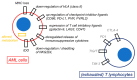
The potential of the immune system to eradicate leukemic cells has been consistently demonstrated by the Graft vs. Leukemia effect occurring after allo-HSCT and in the context of donor leukocyte infusions. Various immunotherapeutic approaches, ranging from the use of antibodies, antibody-drug conjugates, bispecific T-cell engagers, chimeric antigen receptor (CAR) T-cells, and therapeutic infusions of NK cells, are thus currently being tested with promising, yet conflicting, results. This review will concentrate on various types of immunotherapies in preclinical and clinical development, from the point of view of a clinical hematologist. The most promising therapies for clinical translation are the use of bispecific T-cell engagers and CAR-T cells aimed at lineage-restricted antigens, where overall responses (ORR) ranging from 20 to 40% can be achieved in a small series of heavily pretreated patients affected by refractory or relapsing leukemia. Toxicity consists mainly in the occurrence of cytokine-release syndrome, which is mostly manageable with step-up dosing, the early use of cytokine-blocking agents and corticosteroids, and myelosuppression. Various cytokine-enhanced natural killer products are also being tested, mainly as allogeneic off-the-shelf therapies, with a good tolerability profile and promising results (ORR: 20-37.5% in small trials). The in vivo activation of T lymphocytes and NK cells via the inhibition of their immune checkpoints also yielded interesting, yet limited, results (ORR: 33-59%) but with an increased risk of severe Graft vs. Host disease in transplanted patients. Therefore, there are still several hurdles to overcome before the widespread clinical use of these novel compounds.
Keywords: NK cells; T lymphocytes; acute myeloid leukemia; bioengineering; bispecific antibodies; chimeric antigen receptor cells; dual-affinity retargeting antibodies; immune checkpoint inhibitors; immune escape; immunotherapy.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Natural Killer Immunotherapy for Minimal Residual Disease Eradication Following Allogeneic Hematopoietic Stem Cell Transplantation in Acute Myeloid Leukemia.Int J Mol Sci. 2019 Apr 26;20(9):2057. doi: 10.3390/ijms20092057. Int J Mol Sci. 2019. PMID: 31027331 Free PMC article. Review.
-
Transforming CLL management with immunotherapy: Investigating the potential of CAR T-cells and bispecific antibodies.Semin Hematol. 2024 Apr;61(2):119-130. doi: 10.1053/j.seminhematol.2024.01.001. Epub 2024 Jan 5. Semin Hematol. 2024. PMID: 38290860 Review.
-
Immunotherapy for myelodysplastic syndrome and acute myeloid leukemia: where do we stand?Expert Rev Hematol. 2023 Jul-Dec;16(11):819-834. doi: 10.1080/17474086.2023.2268273. Epub 2023 Nov 17. Expert Rev Hematol. 2023. PMID: 37819154 Review.
-
Immune-Based Therapeutic Interventions for Acute Myeloid Leukemia.Cancer Treat Res. 2022;183:225-254. doi: 10.1007/978-3-030-96376-7_8. Cancer Treat Res. 2022. PMID: 35551662
-
Anticancer traits of chimeric antigen receptors (CARs)-Natural Killer (NK) cells as novel approaches for melanoma treatment.BMC Cancer. 2022 Nov 25;22(1):1220. doi: 10.1186/s12885-022-10320-0. BMC Cancer. 2022. PMID: 36434591 Free PMC article.
Cited by
-
Global assessment of leukemia care quality: insights from the quality of care index (QCI) from 1990 to 2021.EClinicalMedicine. 2024 Dec 17;79:102996. doi: 10.1016/j.eclinm.2024.102996. eCollection 2025 Jan. EClinicalMedicine. 2024. PMID: 39802300 Free PMC article.
-
Prognostic value of natural killer T cell related genes in acute myeloid leukemia.Cancer Cell Int. 2025 Apr 13;25(1):143. doi: 10.1186/s12935-025-03779-x. Cancer Cell Int. 2025. PMID: 40223063 Free PMC article.
-
C-type lectin-like molecule-1 as a diagnostic, prognostic, and therapeutic marker in leukemia.Mol Biol Rep. 2025 May 16;52(1):464. doi: 10.1007/s11033-025-10527-x. Mol Biol Rep. 2025. PMID: 40379983 Review.
References
-
- Khoury J.D., Solary E., Abla O., Akkari Y., Alaggio R., Apperley J.F., Bejar R., Berti E., Busque L., Cjan J.K.C., et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–1719. doi: 10.1038/s41375-022-01613-1. - DOI - PMC - PubMed
-
- Arber D.A., Orazi A., Hasserjian R.P., Borowitz M.J., Calvo K.R., Kvasnicka H.M., Wang S.A., Bagg A., Barbui T., Branford S., et al. International Consensus Classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical and genomic data. Blood. 2022;140:1200–1228. doi: 10.1182/blood.2022015850. - DOI - PMC - PubMed
-
- Dohner H., Wei A.H., Appelbaum F.R., Craddock C., DiNardo C.D., Dombret H., Ebert B.L., Fenaux P., Godley L.A., Hasserjian R.P., et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

