Liver Transplantation for Hepatocellular Carcinoma in the Era of Immune Checkpoint Inhibitors
- PMID: 39001436
- PMCID: PMC11240403
- DOI: 10.3390/cancers16132374
Liver Transplantation for Hepatocellular Carcinoma in the Era of Immune Checkpoint Inhibitors
Abstract
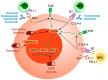
Hepatocellular carcinoma (HCC) remains the leading oncological indication for liver transplantation (LT), with evolving and broadened inclusion criteria. Immune checkpoint inhibitors (ICIs) gained a central role in systemic HCC treatment and showed potential in the peri-transplant setting as downstaging/bridging therapy before LT or as a treatment for HCC recurrence following LT. However, the antagonistic mechanisms of action between ICIs and immunosuppressive drugs pose significant challenges, particularly regarding the risk of acute rejection (AR). This review analyzes the main signaling pathways targeted by ICI therapies and summarizes current studies on ICI therapy before and after LT. The literature on this topic is limited and highly heterogeneous, precluding definitive evidence-based conclusions. The use of ICIs before LT appears promising, provided that a sufficient wash-out period is implemented. In contrast, the results of post-LT ICI therapy do not support its wide clinical application due to high AR rates and overall poor response to treatment. In the future, modern graft preservation techniques might support the selection of good ICI responders, but data from high-level studies are urgently needed.
Keywords: acute rejection; hepatocellular carcinoma; immune checkpoint inhibitors; immunotherapy; liver transplantation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., Montalto F., Ammatuna M., Morabito A., Gennari L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. - DOI - PubMed
-
- Mazzaferro V., Llovet J.M., Miceli R., Bhoori S., Schiavo M., Mariani L., Camerini T., Roayaie S., Schwartz M.E., Grazi G.L., et al. Predicting Survival after Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. - DOI - PubMed
-
- Mazzaferro V., Sposito C., Zhou J., Pinna A.D., De Carlis L., Fan J., Cescon M., Di Sandro S., Yi-feng H., Lauterio A., et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death after Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128–139. doi: 10.1053/j.gastro.2017.09.025. - DOI - PubMed
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

