Impact of Neoadjuvant Treatment on Body Composition in Patients with Locally Advanced Gastric Cancer
- PMID: 39001470
- PMCID: PMC11240361
- DOI: 10.3390/cancers16132408
Impact of Neoadjuvant Treatment on Body Composition in Patients with Locally Advanced Gastric Cancer
Abstract
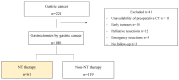
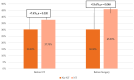
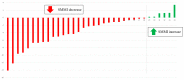
Neoadjuvant chemotherapy (NT) followed by radical surgery is the standard treatment for locally advanced gastric cancer (GC). The incidence of sarcopenia in upper gastrointestinal tract malignancies is very high, and it may be increased after NT. This study aimed to evaluate the impact of NT on body composition. A retrospective study of patients with locally advanced GC undergoing gastrectomy who had received NT in a tertiary hospital between 2012 and 2019 was conducted. CT measured the skeletal muscle index, total psoas area, and visceral and subcutaneous adipose tissue before and after NT. Of the 180 gastrectomies for GC, 61 patients received NT. During NT, changes in body composition were observed with a decrease in the skeletal muscle mass index (SMMI -2.5%; p < 0.001), and these changes were significantly greater in men (SMMI -10.55%). Before surgery, patients who received NT presented 15% more sarcopenia than those without NT (p = 0.048). In conclusion, patients with locally advanced gastric cancer who receive NT have significant changes in body composition during chemotherapy. These changes, which are at the expense of a loss of muscle mass, lead to an increased incidence of pre-surgical sarcopenia.
Keywords: cancer; gastrectomy; gastric; neoadjuvant; sarcopenia.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J.H., Nicolson M., Scarffe J.H., Lofts F.J., Falk S.J., Iveson T.J., et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. - DOI - PubMed
-
- Al-Batran S.E., Homann N., Pauligk C., Goetze T.O., Meiler J., Kasper S., Luley K.B., Trojan J., Martens U.M., Reinacher-Schick A., et al. Perioperative Chemotherapy with Fluorouracil Plus Leucovorin, Oxaliplatin, and Docetaxel versus Fluorouracil or Capecitabine Plus Cisplatin and Epirubicin for Locally Advanced, Resectable Gastric or Gastro-Oesophageal Junction Adenocarcinoma (FLOT4): A Randomised, Phase 2/3 Trial. Lancet. 2019;393:1948–1957. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

