Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development
- PMID: 39001482
- PMCID: PMC11240765
- DOI: 10.3390/cancers16132420
Antibody-Drug Conjugates in Urothelial Cancer: From Scientific Rationale to Clinical Development
Abstract
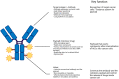
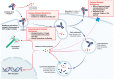
Antibody-drug conjugates (ADCs) have been a significant advancement in cancer therapy, particularly for urothelial cancer (UC). These innovative treatments, originally developed for hematological malignancies, use target-specific monoclonal antibodies linked to potent cytotoxic agents. This rational drug design efficiently delivers cancer cell-killing agents to cells expressing specific surface proteins, which are abundant in UC owing to their high antigen expression. UC is an ideal candidate for ADC therapy, as it enhances on-target efficacy while mitigating systemic toxicity. In recent years, considerable progress has been made in understanding the biology and mechanisms of tumor progression in UC. However, despite the introduction of immune checkpoint inhibitors, advanced UC is characterized by rapid progression and poor survival rates. Targeted therapies that have been developed include the anti-nectin 4 ADC enfortumab vedotin and the fibroblast growth factor receptor inhibitor erdafitinib. Enfortumab vedotin has shown efficacy in prospective studies in patients with advanced UC, alone and in combination with pembrolizumab. The anti-Trop-2 ADC sacituzumab govitecan has also demonstrated effectiveness in single-armed studies. This review highlights the mechanism of action of ADCs, their application in mono- and combination therapies, primary mechanisms of resistance, and future perspectives for their clinical use in UC treatment. ADCs have proven to be an increasingly vital component of the therapeutic landscape for urothelial carcinoma, filling a gap in the treatment of this progressive disease.
Keywords: ADC; ADC resistance mechanism; antibody-drug conjugates; enfortumab vedotin; urothelial carcinoma.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program Cancer Stat Facts: Bladder Cancer. [(accessed on 27 April 2023)]; Available online: https://seer.cancer.gov/statfacts/html/urinb.html.
-
- Witjes J.A., Bruins H.M., Cathomas R., Compérat E.M., Cowan N.C., Gakis G., Hernández V., Espinós E.L., Lorch A., Neuzillet Y. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2020 guidelines. Eur. Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. - DOI - PubMed
-
- Proietti F., Flammia R.S., Licari L.C., Bologna E., Bove A.M., Brassetti A., Tuderti G., Mastroianni R., Tu-fano A., Simone G., et al. Impacts of Neoadjuvant Chemotherapy on Perioperative Outcomes in Patients with Bladder Cancer Treated with Radical Cystectomy: A Single High-Volume Center Experience. J. Pers. Med. 2024;14:212. doi: 10.3390/jpm14020212. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

