Breast Cancer Patient's Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II-III Disease
- PMID: 39001483
- PMCID: PMC11240707
- DOI: 10.3390/cancers16132421
Breast Cancer Patient's Outcomes after Neoadjuvant Chemotherapy and Surgery at 5 and 10 Years for Stage II-III Disease
Abstract
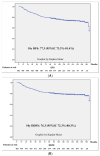
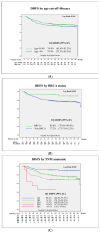
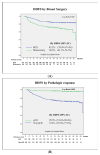
Introduction: Neoadjuvant chemotherapy in breast cancer offers the possibility to facilitate breast and axillary surgery; it is a test of chemosensibility in vivo with significant prognostic value and may be used to tailor adjuvant treatment according to the response. Material and Methods: A retrospective single-institution cohort of 482 stage II and III breast cancer patients treated with neoadjuvant chemotherapy based on anthracycline and taxans, plus antiHEr2 in Her2-positive cases, was studied. Survival was calculated at 5 and 10 years. Kaplan-Meier curves with a log-rank test were calculated for differences according to age, BRCA status, menopausal status, TNM, pathological and molecular surrogate subtype, 20% TIL cut-off, surgical procedure, response to chemotherapy and the presence of vascular invasion. Results: The pCR rate was 25.3% and was greater in HER2 (51.3%) and TNBC (31.7%) and in BRCA carriers (41.9%). The factors independently related to patient survival were pathology and molecular surrogate subtype, type of surgery, response to NACT and vascular invasion. BRCA status was a protective prognostic factor without reaching statistical significance, with an HR 0.5 (95%CI 0.1-1.4). Mastectomy presented a double risk of distant recurrence compared to breast-conservative surgery (BCS), supporting BCS as a safe option after NACT. After a mean follow-up of 126 (SD 43) months, luminal tumors presented a substantial difference in survival rates calculated at 5 or 10 years (81.2% compared to 74.7%), whereas that for TNBC was 75.3 and 73.5, respectively. The greatest difference was seen according to the response in patients with pCR, who exhibited a 10 years DDFS of 95.5% vs. 72.4% for those patients without pCR, p < 0001. This difference was especially meaningful in TNBC: the 10 years DDFS according to an RCB of 0 to 3 was 100%, 80.6%, 69% and 49.2%, respectively, p < 0001. Patients with a particularly poor prognosis were those with lobular carcinomas, with a 10 years DDFS of 42.9% vs. 79.7% for ductal carcinomas, p = 0.001, and patients with vascular invasion at the surgical specimen, with a 10 years DDFS of 59.2% vs. 83.6% for those patients without vascular invasion, p < 0.001. Remarkably, BRCA carriers presented a longer survival, with an estimated 10 years DDFS of 89.6% vs. 77.2% for non-carriers, p = 0.054. Conclusions: Long-term outcomes after neoadjuvant chemotherapy can help patients and clinicians make well-informed decisions.
Keywords: breast cancer; neoadjuvant chemotherapy; prognostic factors; survival; well-informed decision making.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bonadonna G., Veronesi U., Brambilla C., Ferrari L., Luini A., Greco M., Bartoli C., de Yoldi G.C., Zucali R., Rilke F., et al. Primary chemotherapy to avoid Mastectomy in tumors with diameters of three centimeters or more. J. Natl. Cancer Inst. 1990;82:1539–1545. doi: 10.1093/jnci/82.19.1539. - DOI - PubMed
-
- Wolmark N., Wang J., Mamounas E., Bryant J., Fisher B. Preoperative Chemotherapy in patients with operable Breast Cancer: Nine-years results from National Surgical Adjuvant Breast and Bowel Project B-18. J. Natl. Cancer Inst. Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. - DOI - PubMed
-
- Goldhirsch A., Winer E.P., Coates A.S., Gelber R.D., Piccart-Gebhart M., Thürlimann B., Senn H.J., Albain K.S., André F., Bergh J., et al. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann. Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. - DOI - PMC - PubMed
-
- Tinterri C., Barbieri E., Sagona A., Bottini A., Canavese G., Gentile D. De-Escalation Surgery in cT3-4 Breast Cancer Patients after Neoadjuvant Therapy: Predictors of Breast Conservation and Comparison of Long-Term Oncological Outcomes with Mastectomy. Cancers. 2024;16:1169. doi: 10.3390/cancers16061169. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

