Transcript Markers from Urinary Extracellular Vesicles for Predicting Risk Reclassification of Prostate Cancer Patients on Active Surveillance
- PMID: 39001515
- PMCID: PMC11240337
- DOI: 10.3390/cancers16132453
Transcript Markers from Urinary Extracellular Vesicles for Predicting Risk Reclassification of Prostate Cancer Patients on Active Surveillance
Abstract
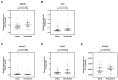
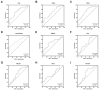
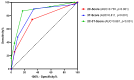
Serum prostate-specific antigen (PSA), its derivatives, and magnetic resonance tomography (MRI) lack sufficient specificity and sensitivity for the prediction of risk reclassification of prostate cancer (PCa) patients on active surveillance (AS). We investigated selected transcripts in urinary extracellular vesicles (uEV) from PCa patients on AS to predict PCa risk reclassification (defined by ISUP 1 with PSA > 10 ng/mL or ISUP 2-5 with any PSA level) in control biopsy. Before the control biopsy, urine samples were prospectively collected from 72 patients, of whom 43% were reclassified during AS. Following RNA isolation from uEV, multiplexed reverse transcription, and pre-amplification, 29 PCa-associated transcripts were quantified by quantitative PCR. The predictive ability of the transcripts to indicate PCa risk reclassification was assessed by receiver operating characteristic (ROC) curve analyses via calculation of the area under the curve (AUC) and was then compared to clinical parameters followed by multivariate regression analysis. ROC curve analyses revealed a predictive potential for AMACR, HPN, MALAT1, PCA3, and PCAT29 (AUC = 0.614-0.655, p < 0.1). PSA, PSA density, PSA velocity, and MRI maxPI-RADS showed AUC values of 0.681-0.747 (p < 0.05), with accuracies for indicating a PCa risk reclassification of 64-68%. A model including AMACR, MALAT1, PCAT29, PSA density, and MRI maxPI-RADS resulted in an AUC of 0.867 (p < 0.001) with a sensitivity, specificity, and accuracy of 87%, 83%, and 85%, respectively, thus surpassing the predictive power of the individual markers. These findings highlight the potential of uEV transcripts in combination with clinical parameters as monitoring markers during the AS of PCa.
Keywords: active surveillance; biomarker; liquid biopsy; monitoring; prediction; prostate cancer; quantitative PCR; risk reclassification; transcripts; urinary extracellular vesicles.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Sayyid R.K., Benton J.Z., Reed W.C., Woodruff P., Terris M.K., Wallis C.J.D., Klaassen Z. Prostate cancer mortality rates in low- and favorable intermediate-risk active surveillance patients: A population-based competing risks analysis. World J. Urol. 2023;41:93–99. doi: 10.1007/s00345-022-04228-4. - DOI - PubMed
-
- Cornford P., van den Bergh R.C.N., Briers E., Van den Broeck T., Brunckhorst O., Darraugh J., Eberli D., De Meerleer G., De Santis M., Farolfi A., et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024. in press . - DOI - PubMed
-
- Bokhorst L.P., Valdagni R., Rannikko A., Kakehi Y., Pickles T., Bangma C.H., Roobol M.J., for the PRIAS study group A Decade of Active Surveillance in the PRIAS Study: An Update and Evaluation of the Criteria Used to Recommend a Switch to Active Treatment. Eur. Urol. 2016;70:954–960. doi: 10.1016/j.eururo.2016.06.007. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

