The Role of Immunotherapy in the Management of Esophageal Cancer in Patients Treated with Neoadjuvant Chemoradiation: An Analysis of the National Cancer Database
- PMID: 39001522
- PMCID: PMC11240428
- DOI: 10.3390/cancers16132460
The Role of Immunotherapy in the Management of Esophageal Cancer in Patients Treated with Neoadjuvant Chemoradiation: An Analysis of the National Cancer Database
Abstract
Background: The current National Comprehensive Cancer Network advises neoadjuvant chemoradiotherapy followed by surgery for locally advanced cases of esophageal cancer. The role of immunotherapy in this context is under heavy investigation.
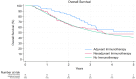
Methods: Patients with esophageal adenocarcinoma were identified in the National Cancer Database (NCDB) from 2004 to 2019. Three groups were generated as follows: (a) no immunotherapy, (b) neoadjuvant immunotherapy, and (c) adjuvant immunotherapy. Overall survival was evaluated using the Kaplan-Meier method and Cox proportional hazard analysis, adjusting for previously described risk factors for mortality.
Results: Of the total 14,244 patients diagnosed with esophageal adenocarcinoma who received neoadjuvant chemoradiation, 14,065 patients did not receive immunotherapy, 110 received neoadjuvant immunotherapy, and 69 received adjuvant immunotherapy. When adjusting for established risk factors, adjuvant immunotherapy was associated with significantly improved survival compared to no immunotherapy and neoadjuvant immunotherapy during a median follow-up period of 35.2 months. No difference was noted among patients who received no immunotherapy vs. neoadjuvant immunotherapy in the same model.
Conclusions: In this retrospective analysis of the NCDB, receiving adjuvant immunotherapy offered a significant survival advantage compared to no immunotherapy and neoadjuvant immunotherapy in the treatment of esophageal adenocarcinoma. The addition of neoadjuvant immunotherapy to patients treated with neoadjuvant chemoradiation did not improve survival in this cohort. Further studies are warranted to investigate the long-term outcomes of immunotherapy in esophageal cancer.
Keywords: adjuvant immunotherapy; esophageal cancer; immunotherapy; neoadjuvant immunotherapy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ajani J.A., D’amico T.A., Bentrem D.J., Cooke D., Corvera C., Das P., Enzinger P.C., Enzler T., Farjah F., Gerdes H., et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023;21:393–422. doi: 10.6004/jnccn.2023.0019. - DOI - PubMed
LinkOut - more resources
Full Text Sources