Association of Serum Proteases and Acute Phase Factors Levels with Survival Outcomes in Patients with Colorectal Cancer
- PMID: 39001534
- PMCID: PMC11240471
- DOI: 10.3390/cancers16132471
Association of Serum Proteases and Acute Phase Factors Levels with Survival Outcomes in Patients with Colorectal Cancer
Abstract
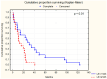
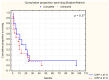
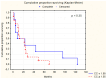
Colorectal cancer (CRC) represents a substantial burden on global healthcare, contributing to significant morbidity and mortality worldwide. Despite advances in screening methodologies, its incidence remains high, necessitating continued efforts in early detection and treatment. Neoplastic invasion and metastasis are primary determinants of CRC lethality, emphasizing the urgency of understanding underlying mechanisms to develop effective therapeutic strategies. This study aimed to explore the potential of serum biomarkers in predicting survival outcomes in CRC patients, with a focus on cathepsin B (CB), leukocytic elastase (LE), total sialic acid (TSA), lipid-associated sialic acid (LASA), antitrypsin activity (ATA), C-reactive protein (CRP), and cystatin C (CC). We recruited 185 CRC patients and 35 healthy controls, assessing demographic variables, tumor characteristics, and 7 serum biomarker levels, including (1) CB, (2) LE, (3) TSA, (4) LASA, (5) ATA, (6) CRP, and (7) CC. Statistical analyses included ANOVA with Tukey's post hoc tests and MANOVA for continuous variables. Student's t-test was used for dependent samples, while non-parametric tests like Mann-Whitney U and Wilcoxon signed-rank tests were applied for variables deviating from the normal distribution. Categorical variables were assessed using chi-square and Kruskal-Wallis tests. Spearman's rank correlation coefficient was utilized to examine variable correlations. Survival analysis employed the Kaplan-Meier method with a log-rank test for comparing survival times between groups. Significant associations were observed between CB (p = 0.04), LE (p = 0.01), and TSA (p = 0.008) levels and survival outcomes in CRC patients. Dukes' classification stages also showed a significant correlation with survival (p = 0.001). However, no significant associations were found for LASA, ATA, CRP, and CC. Multivariate analysis of LE, TSA, and ATA demonstrated a notable correlation with survival (p = 0.041), notwithstanding ATA's lack of significance in univariate analysis (p = 0.13). CB, LE, and TSA emerged as promising diagnostic markers with prognostic value in CRC, potentially aiding in early diagnosis and treatment planning. Further research is needed to validate these findings and explore additional prognostic indicators.
Keywords: C-reactive protein; acute phase factors; cathepsin B; colorectal cancer; leukocytic elastase; sialic acid; survival analysis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Diagnostic usefulness of selected proteases and acute phase factors in patients with colorectal adenocarcinoma.World J Gastroenterol. 2021 Oct 21;27(39):6673-6688. doi: 10.3748/wjg.v27.i39.6673. World J Gastroenterol. 2021. PMID: 34754160 Free PMC article.
-
Multivariate analysis of the prognostic value of CEA and CA 19-9 serum levels in colorectal cancer.Anticancer Res. 2000 Nov-Dec;20(6D):5195-8. Anticancer Res. 2000. PMID: 11326694
-
Clinical significance of preoperative serum sialic acid levels in colorectal cancer: utility in the detection of patients at high risk of tumor recurrence.Int J Biol Markers. 2004 Jan-Mar;19(1):38-45. doi: 10.1177/172460080401900105. Int J Biol Markers. 2004. PMID: 15077925
-
Total and lipid-bound plasma sialic acid as diagnostic markers in colorectal cancer patients: correlation with cathepsin B expression in progression to Dukes stage.J Exp Ther Oncol. 2006;5(3):223-9. J Exp Ther Oncol. 2006. PMID: 16528972
-
Preoperative serum levels of CEA and CA 19-9 and their prognostic significance in colorectal carcinoma.Anticancer Res. 1997 Jul-Aug;17(4B):2935-8. Anticancer Res. 1997. PMID: 9329568
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

