Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin
- PMID: 39001720
- PMCID: PMC11416330
- DOI: 10.1016/j.jid.2024.04.022
Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin
Abstract
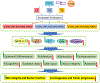
Novel pathways of vitamin D3, lumisterol 3 (L3), and tachysterol 3 (T3) activation have been discovered, initiated by CYP11A1 and/or CYP27A1 in the case of L3 and T3. The resulting hydroxymetabolites enhance protection of skin against DNA damage and oxidative stress; stimulate keratinocyte differentiation; exert anti-inflammatory, antifibrogenic, and anticancer activities; and inhibit cell proliferation in a structure-dependent manner. They act on nuclear receptors, including vitamin D receptor, aryl hydrocarbon receptor, LXRα/β, RAR-related orphan receptor α/γ, and peroxisome proliferator-activated receptor-γ, with selectivity defined by their core structure and distribution of hydroxyl groups. They can activate NRF2 and p53 and inhibit NF-κB, IL-17, Shh, and Wnt/β-catenin signaling. Thus, they protect skin integrity and physiology.
Keywords: Lumisterol; Nuclear receptors; Skin; Tachysterol; Vitamin D receptors.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures

References
-
- Bikle D, Christakos S. New aspects of vitamin D metabolism and action - addressing the skin as source and target. Nat Rev Endocrinol 2020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

