Genetic deletion or pharmacologic inhibition of histone deacetylase 6 protects the heart against ischaemia/reperfusion injury by limiting tumour necrosis factor alpha-induced mitochondrial injury in experimental diabetes
- PMID: 39001869
- PMCID: PMC11472425
- DOI: 10.1093/cvr/cvae144
Genetic deletion or pharmacologic inhibition of histone deacetylase 6 protects the heart against ischaemia/reperfusion injury by limiting tumour necrosis factor alpha-induced mitochondrial injury in experimental diabetes
Abstract
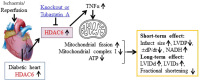
Aims: The histone deacetylase 6 (HDAC6) inhibitor, tubastatin A (TubA), reduces myocardial ischaemia/reperfusion injury (MIRI) in type 1 diabetic rats. It remains unclear whether HDAC6 regulates MIRI in type 2 diabetic animals. Diabetes augments the activity of HDAC6 and the generation of tumour necrosis factor alpha (TNF-α) and impairs mitochondrial complex I (mCI). Here, we examined how HDAC6 regulates TNF-α production, mCI activity, mitochondria, and cardiac function in type 1 and type 2 diabetic mice undergoing MIRI.
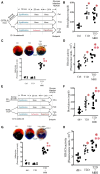
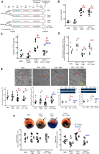
Methods and results: HDAC6 knockout, streptozotocin-induced type 1 diabetic, and obese type 2 diabetic db/db mice underwent MIRI in vivo or ex vivo in a Langendorff-perfused system. We found that MIRI and diabetes additively augmented myocardial HDAC6 activity and generation of TNF-α, along with cardiac mitochondrial fission, low bioactivity of mCI, and low production of adenosine triphosphate. Importantly, genetic disruption of HDAC6 or TubA decreased TNF-α levels, mitochondrial fission, and myocardial mitochondrial nicotinamide adenine dinucleotide levels in ischaemic/reperfused diabetic mice, concomitant with augmented mCI activity, decreased infarct size, and improved cardiac function. Moreover, HDAC6 knockout or TubA treatment decreased left ventricular dilation and improved cardiac systolic function 28 days after MIRI. H9c2 cardiomyocytes with and without HDAC6 knockdown were subjected to hypoxia/reoxygenation injury in the presence of high glucose. Hypoxia/reoxygenation augmented HDAC6 activity and TNF-α levels and decreased mCI activity. These negative effects were blocked by HDAC6 knockdown.
Conclusion: HDAC6 is an essential negative regulator of MIRI in diabetes. Genetic deletion or pharmacologic inhibition of HDAC6 protects the heart from MIRI by limiting TNF-α-induced mitochondrial injury in experimental diabetes.
Keywords: Histone deacetylase 6; Ischaemia/reperfusion; Mitochondria; Tumour necrosis factor alpha; Type 1 diabetes; Type 2 diabetes.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared.
Figures





Comment in
-
Histone deacetylase 6 suppression protects from myocardial ischaemia-reperfusion injury in diabetes: insights from genetic deletion and pharmacological inhibition.Cardiovasc Res. 2024 Oct 14;120(12):1369-1371. doi: 10.1093/cvr/cvae145. Cardiovasc Res. 2024. PMID: 39082188 No abstract available.
References
-
- Larsson SC, Wallin A, Hakansson N, Stackelberg O, Back M, Wolk A. Type 1 and type 2 diabetes mellitus and incidence of seven cardiovascular diseases. Int J Cardiol 2018;262:66–70. - PubMed
-
- Lin YH, Major JL, Liebner T, Hourani Z, Travers JG, Wennersten SA, Haefner KR, Cavasin MA, Wilson CE, Jeong MY, Han Y, Gotthardt M, Ferguson SK, Ambardekar AV, Lam MP, Choudhary C, Granzier HL, Woulfe KC, McKinsey TA. HDAC6 modulates myofibril stiffness and diastolic function of the heart. J Clin Invest 2022;132:e148333. - PMC - PubMed
-
- Kalinski AL, Kar AN, Craver J, Tosolini AP, Sleigh JN, Lee SJ, Hawthorne A, Brito-Vargas P, Miller-Randolph S, Passino R, Shi L, Wong VSC, Picci C, Smith DS, Willis DE, Havton LA, Schiavo G, Giger RJ, Langley B, Twiss JL. Deacetylation of Miro1 by HDAC6 blocks mitochondrial transport and mediates axon growth inhibition. J Cell Biol 2019;218:1871–1890. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

