Selection with two alleles of X-linkage and its application to the fitness component analysis of OdsH in Drosophila
- PMID: 39001870
- PMCID: PMC11537805
- DOI: 10.1093/g3journal/jkae157
Selection with two alleles of X-linkage and its application to the fitness component analysis of OdsH in Drosophila
Abstract
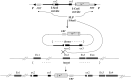
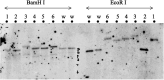
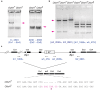
In organisms with the XY sex-determination system, there is an imbalance in the inheritance and transmission of the X chromosome between males and females. Unlike an autosomal allele, an X-linked recessive allele in a female will have phenotypic effects on its male counterpart. Thus, genes located on the X chromosome are of particular interest to researchers in molecular evolution and genetics. Here we present a model for selection with two alleles of X-linkage to understand fitness components associated with genes on the X chromosome. We apply this model to the fitness analysis of an X-linked gene, OdsH (16D), in the fruit fly Drosophila melanogaster. The function of OdsH is involved in sperm production and the gene is rapidly evolving under positive selection. Using site-directed gene targeting, we generated functional and defective OdsH variants tagged with the eye-color marker gene white. We compare the allele frequency changes of the two OdsH variants, each directly competing against a wild-type OdsH allele in concurrent but separate experimental populations. After 20 generations, the two genetically modified OdsH variants displayed a 40% difference in allele frequencies, with the functional OdsH variant demonstrating an advantage over the defective variant. Using maximum likelihood estimation, we determined the fitness components associated with the OdsH alleles in males and females. Our analysis revealed functional aspects of the fitness determinants associated with OdsH, and that sex-specific fertility and viability consequences both contribute to selection on an X-linked gene.
Keywords: OdsH; X-linkage; fitness components; selection; sex bias; two alleles.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Genetics Society of America.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures



References
-
- Barclay HJ, Fitz-Earle M. 1988. Maximum likelihood estimates of fitness in iteroparous species population replacement experiments using compound chromosomes. Genome. 30(1):83–88. doi: 10.1139/g88-014 - DOI
-
- Beckenbach A. 1983. Fitness analysis of the “sex-ratio” polymorphism in experimental populations of Drosophila pseudoobscura. Am Nat. 121(5):630–648. doi: 10.1086/284091 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
