Toll-like receptor 4 mutation mitigates gut microbiota-mediated hypertensive kidney injury
- PMID: 39002869
- PMCID: PMC11287947
- DOI: 10.1016/j.phrs.2024.107303
Toll-like receptor 4 mutation mitigates gut microbiota-mediated hypertensive kidney injury
Abstract
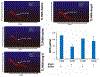
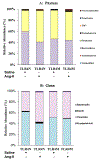
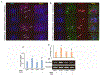
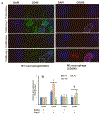
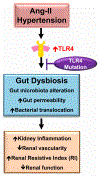
Hypertension-associated dysbiosis is linked to several clinical complications, including inflammation and possible kidney dysfunction. Inflammation and TLR4 activation during hypertension result from gut dysbiosis-related impairment of intestinal integrity. However, the contribution of TLR4 in kidney dysfunction during hypertension-induced gut dysbiosis is unclear. We designed this study to address this knowledge gap by utilizing TLR4 normal (TLR4N) and TLR4 mutant (TLR4M) mice. These mice were infused with high doses of Angiotensin-II for four weeks to induce hypertension. Results suggest that Ang-II significantly increased renal arterial resistive index (RI), decreased renal vascularity, and renal function (GFR) in TLR4N mice compared to TLR4M. 16 S rRNA sequencing analysis of gut microbiome revealed that Ang-II-induced hypertension resulted in alteration of Firmicutes: Bacteroidetes ratio in the gut of both TLR4N and TLR4M mice; however, it was not comparably rather differentially. Additionally, Ang-II-hypertension decreased the expression of tight junction proteins and increased gut permeability, which were more prominent in TLR4N mice than in TLR4M mice. Concomitant with gut hyperpermeability, an increased bacterial component translocation to the kidney was observed in TLR4N mice treated with Ang-II compared to TLR4N plus saline. Interestingly, microbiota translocation was mitigated in Ang-II-hypertensive TLR4M mice. Furthermore, Ang-II altered the expression of inflammatory (IL-1β, IL-6) and anti-inflammatory IL-10) markers, and extracellular matrix proteins, including MMP-2, -9, -14, and TIMP-2 in the kidney of TLR4N mice, which were blunted in TLR4M mice. Our data demonstrate that ablation of TLR4 attenuates hypertension-induced gut dysbiosis resulting in preventing gut hyperpermeability, bacterial translocation, mitigation of renal inflammation and alleviation of kidney dysfunction.
Keywords: Gut dysbiosis; Hypertension; Kidney function; MMP; TIMP; Tight junction.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Townsend RR, Taler SJ, Management of hypertension in chronic kidney disease, Nat. Rev. Nephrol 11 (9) (2015) 555–563. - PubMed
-
- Hamrahian SM, Falkner B, Hypertension in chronic kidney disease, Adv. Exp. Med. Biol 956 (2017) 307–325. - PubMed
-
- Duni A, Dounousi E, Pavlakou P, Eleftheriadis T, Liakopoulos V, Hypertension in chronic kidney disease: novel insights, Curr. Hypertens. Rev 16 (1) (2020) 45–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

