Building on the clinical applicability of ctDNA analysis in non-metastatic pancreatic ductal adenocarcinoma
- PMID: 39003322
- PMCID: PMC11246447
- DOI: 10.1038/s41598-024-67235-y
Building on the clinical applicability of ctDNA analysis in non-metastatic pancreatic ductal adenocarcinoma
Abstract
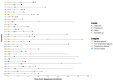
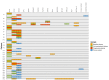
Pancreatic ductal adenocarcinoma represents one of the solid tumors showing the worst prognosis worldwide, with a high recurrence rate after adjuvant or neoadjuvant therapy. Circulating tumor DNA analysis raised as a promising non-invasive tool to characterize tumor genomics and to assess treatment response. In this study, surgical tumor tissue and sequential blood samples were analyzed by next-generation sequencing and were correlated with clinical and pathological characteristics. Thirty resectable/borderline pancreatic ductal adenocarcinoma patients treated at the Hospital Universitario de Navarra were included. Circulating tumoral DNA sequencing identified pathogenic variants in KRAS and TP53, and in other cancer-associated genes. Pathogenic variants at diagnosis were detected in patients with a poorer outcome, and were correlated with response to neoadjuvant therapy in borderline pancreatic ductal adneocarcinoma patients. Higher variant allele frequency at diagnosis was associated with worse prognosis, and thesum of variant allele frequency was greater in samples at progression. Our results build on the potential value of circulating tumor DNA for non-metastatic pancreatic ductal adenocarcinoma patients, by complementing tissue genetic information and as a non-invasive tool for treatment decision. Confirmatory studies are needed to corroborate these findings.
Keywords: Biomarkers; Gastrointestinal neoplasms; Genomics; Liquid biopsy; Precision medicine.
© 2024. The Author(s).
Conflict of interest statement
IL, AEH, SM, IGB, DGS, EM, DG and AV declare no conflict of interest; MA has been involved as a consultant for advisory roles with Amgen, BMS, MSD, Lilly and Servier; HA has been involved as a consultant for advisory roles from Astra Zeneca and for trial coordination from Ferrer Farma; NC: has received speaker honoraria from Roche and Pierre Fabre; AL has received speaker honoraria from Pierre-Fabre; APG has received speaker honoraria from Merck Sharp; GAA has received speaker honoraria from ThermoFisher and Roche; IHG has received speaker honoraria from Astra Zeneca; VA has been involved as a consultant for advisory roles and received speaker honoraria from MSD, Bristol, Lilly, Astra-Zeneca and Pierre-Fabre. RV has been involved as a consultant for advisory roles with Servier, Roche and Merck Sharp and has received speaker honoraria from Roche, Amgen, Merck Sharp and Dohme, Astra Zeneca.
Figures


References
MeSH terms
Substances
Grants and funding
- FJC2021-046521-I/Spanish National Agency of Research (AEI)
- CLJUN19010ARAS/Clínico Junior 2019 scholarship from the Spanish Association Against Cancer (AECC)
- CLJUN234885LECU/Clinico Junior 2023 scholarship from the Spanish Association Against Cancer (AECC)
- 0011-1408-2017-000026/Department of Economic Development of Navarre
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

