PP2A-B55 phosphatase counteracts Ki-67-dependent chromosome individualization during mitosis
- PMID: 39003739
- PMCID: PMC11290319
- DOI: 10.1016/j.celrep.2024.114494
PP2A-B55 phosphatase counteracts Ki-67-dependent chromosome individualization during mitosis
Abstract
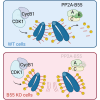
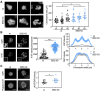
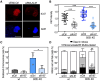
Cell cycle progression is regulated by the orderly balance between kinase and phosphatase activities. PP2A phosphatase holoenzymes containing the B55 family of regulatory B subunits function as major CDK1-counteracting phosphatases during mitotic exit in mammals. However, the identification of the specific mitotic roles of these PP2A-B55 complexes has been hindered by the existence of multiple B55 isoforms. Here, through the generation of loss-of-function genetic mouse models for the two ubiquitous B55 isoforms (B55α and B55δ), we report that PP2A-B55α and PP2A-B55δ complexes display overlapping roles in controlling the dynamics of proper chromosome individualization and clustering during mitosis. In the absence of PP2A-B55 activity, mitotic cells display increased chromosome individualization in the presence of enhanced phosphorylation and perichromosomal loading of Ki-67. These data provide experimental evidence for a regulatory mechanism by which the balance between kinase and PP2A-B55 phosphatase activity controls the Ki-67-mediated spatial organization of the mass of chromosomes during mitosis.
Keywords: B55; CP: Cell biology; CP: Molecular biology; Ki-67; PP2A; PPP2R2A; PPP2R2D; cell division; chromosome clustering; chromosome periphery; mitosis; phosphatase.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

