Multiple transatlantic incursions of highly pathogenic avian influenza clade 2.3.4.4b A(H5N5) virus into North America and spillover to mammals
- PMID: 39003741
- PMCID: PMC11305400
- DOI: 10.1016/j.celrep.2024.114479
Multiple transatlantic incursions of highly pathogenic avian influenza clade 2.3.4.4b A(H5N5) virus into North America and spillover to mammals
Abstract
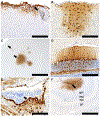
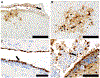
Highly pathogenic avian influenza (HPAI) viruses have spread at an unprecedented scale, leading to mass mortalities in birds and mammals. In 2023, a transatlantic incursion of HPAI A(H5N5) viruses into North America was detected, followed shortly thereafter by a mammalian detection. As these A(H5N5) viruses were similar to contemporary viruses described in Eurasia, the transatlantic spread of A(H5N5) viruses was most likely facilitated by pelagic seabirds. Some of the Canadian A(H5N5) viruses from birds and mammals possessed the PB2-E627K substitution known to facilitate adaptation to mammals. Ferrets inoculated with A(H5N5) viruses showed rapid, severe disease onset, with some evidence of direct contact transmission. However, these viruses have maintained receptor binding traits of avian influenza viruses and were susceptible to oseltamivir and zanamivir. Understanding the factors influencing the virulence and transmission of A(H5N5) in migratory birds and mammals is critical to minimize impacts on wildlife and public health.
Keywords: A(H5N5); CP: Microbiology; HPAI; Sable Island; antiviral susceptibility; avian influenza; clade 2.3.4.4b; contact transmission; ferret model; wildlife transmission.
Crown Copyright © 2024. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
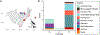


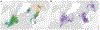
References
-
- Dhingra MS, Artois J, Dellicour S, Lemey P, Dauphin G, Von Dobschuetz S, Van Boeckel TP, Castellan DM, Morzaria S, and Gilbert M (2018). Geographical and Historical Patterns in the Emergences of Novel Highly Pathogenic Avian Influenza (HPAI) H5 and H7 Viruses in Poultry. Front. Vet. Sci 5, 84. 10.3389/fvets.2018.00084. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

