Protocell Effects on RNA Folding, Function, and Evolution
- PMID: 39005057
- PMCID: PMC11308369
- DOI: 10.1021/acs.accounts.4c00174
Protocell Effects on RNA Folding, Function, and Evolution
Abstract
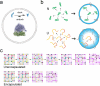
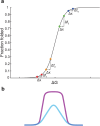
Creating a living system from nonliving matter is a great challenge in chemistry and biophysics. The early history of life can provide inspiration from the idea of the prebiotic "RNA World" established by ribozymes, in which all genetic and catalytic activities were executed by RNA. Such a system could be much simpler than the interdependent central dogma characterizing life today. At the same time, cooperative systems require a mechanism such as cellular compartmentalization in order to survive and evolve. Minimal cells might therefore consist of simple vesicles enclosing a prebiotic RNA metabolism. The internal volume of a vesicle is a distinctive environment due to its closed boundary, which alters diffusion and available volume for macromolecules and changes effective molecular concentrations, among other considerations. These physical effects are mechanistically distinct from chemical interactions, such as electrostatic repulsion, that might also occur between the membrane boundary and encapsulated contents. Both indirect and direct interactions between the membrane and RNA can give rise to nonintuitive, "emergent" behaviors in the model protocell system. We have been examining how encapsulation inside membrane vesicles would affect the folding and activity of entrapped RNA. Using biophysical techniques such as FRET, we characterized ribozyme folding and activity inside vesicles. Encapsulation inside model protocells generally promoted RNA folding, consistent with an excluded volume effect, independently of chemical interactions. This energetic stabilization translated into increased ribozyme activity in two different systems that were studied (hairpin ribozyme and self-aminoacylating RNAs). A particularly intriguing finding was that encapsulation could rescue the activity of mutant ribozymes, suggesting that encapsulation could affect not only folding and activity but also evolution. To study this further, we developed a high-throughput sequencing assay to measure the aminoacylation kinetics of many thousands of ribozyme variants in parallel. The results revealed an unexpected tendency for encapsulation to improve the better ribozyme variants more than worse variants. During evolution, this effect would create a tilted playing field, so to speak, that would give additional fitness gains to already-high-activity variants. According to Fisher's Fundamental Theorem of Natural Selection, the increased variance in fitness should manifest as faster evolutionary adaptation. This prediction was borne out experimentally during in vitro evolution, where we observed that the initially diverse ribozyme population converged more quickly to the most active sequences when they were encapsulated inside vesicles. The studies in this Account have expanded our understanding of emergent protocell behavior, by showing how simply entrapping an RNA inside a vesicle, which could occur spontaneously during vesicle formation, might profoundly affect the evolutionary landscape of the RNA. Because of the exponential dynamics of replication and selection, even small changes to activity and function could lead to major evolutionary consequences. By closely studying the details of minimal yet surprisingly complex protocells, we might one day trace a pathway from encapsulated RNA to a living system.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Woese C. R.The Genetic Code: The Molecular Basis for Genetic Expression; Harper & Row, 1967.

