An Ultrasensitive Biosensor for Probing Subcellular Distribution and Mitochondrial Transport of l-2-Hydroxyglutarate
- PMID: 39005231
- PMCID: PMC11425224
- DOI: 10.1002/advs.202404119
An Ultrasensitive Biosensor for Probing Subcellular Distribution and Mitochondrial Transport of l-2-Hydroxyglutarate
Abstract
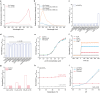
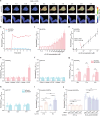
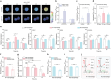
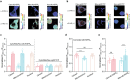
l-2-Hydroxyglutarate (l-2-HG) is a functionally compartmentalized metabolite involved in various physiological processes. However, its subcellular distribution and mitochondrial transport remain unclear owing to technical limitations. In the present study, an ultrasensitive l-2-HG biosensor, sfLHGFRH, composed of circularly permuted yellow fluorescent protein and l-2-HG-specific transcriptional regulator, is developed. The ability of sfLHGFRH to be used for analyzing l-2-HG metabolism is first determined in human embryonic kidney cells (HEK293FT) and macrophages. Then, the subcellular distribution of l-2-HG in HEK293FT cells and the lower abundance of mitochondrial l-2-HG are identified by the sfLHGFRH-supported spatiotemporal l-2-HG monitoring. Finally, the role of the l-glutamate transporter SLC1A1 in mitochondrial l-2-HG uptake is elucidated using sfLHGFRH. Based on the design of sfLHGFRH, another highly sensitive biosensor with a low limit of detection, sfLHGFRL, is developed for the point-of-care diagnosis of l-2-HG-related diseases. The accumulation of l-2-HG in the urine of patients with kidney cancer is determined using the sfLHGFRL biosensor.
Keywords: biosensor; l‐2‐hydroxyglutarate; metabolism; mitochondrial transport; point‐of‐care testing.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
C.G., Z.K., C.M., and P.X. are inventors of patent applications (Chinese patent application no. 2023114979187). The patent was submitted by Shandong University. Other authors declare no relevant conflicts of interest.
Figures



References
-
- Harris A. L., Cell Metab. 2015, 22, 198. - PubMed
MeSH terms
Substances
Grants and funding
- 2019YFA0904800/National Key Research and Development Program of China
- 32270074/National Natural Science Foundation of China
- 32170112/National Natural Science Foundation of China
- 32300029/Youth Program of National Natural Science Foundation of China
- 32300045/Youth Program of National Natural Science Foundation of China
- 2023M742085/China Postdoctoral Science Foundation
- ZR2023QC237/Youth Program of Natural Science Foundation of Shandong Province
- 23-2-1-31-zyyd-jch/Youth Program of Natural Science Foundation of Qingdao City
- QDBSH20230201011/Qingdao Postdoctoral Research Project
- SKLMTFCP-2023-03/State Key Laboratory of Microbial Technology
LinkOut - more resources
Full Text Sources
