Model-Based Analysis of Arsenic Retention by Stimulated Iron Mineral Transformation under Coastal Aquifer Conditions
- PMID: 39005241
- PMCID: PMC11242918
- DOI: 10.1021/acsestwater.4c00134
Model-Based Analysis of Arsenic Retention by Stimulated Iron Mineral Transformation under Coastal Aquifer Conditions
Abstract
A multitude of geochemical processes control the aqueous concentration and transport properties of trace metal contaminants such as arsenic (As) in groundwater environments. Effective As remediation, especially under reducing conditions, has remained a significant challenge. Fe(II) nitrate treatments are a promising option for As immobilization but require optimization to be most effective. Here, we develop a process-based numerical modeling framework to provide an in-depth understanding of the geochemical mechanisms controlling the response of As-contaminated sediments to Fe(II) nitrate treatment. The analyzed data sets included time series from two batch experiments (control vs treatment) and effluent concentrations from a flow-through column experiment. The reaction network incorporates a mixture of homogeneous and heterogeneous reactions affecting Fe redox chemistry. Modeling revealed that the precipitation of the Fe treatment caused a rapid pH decline, which then triggered multiple heterogeneous buffering processes. The model quantifies key processes for effective remediation, including the transfer of aqueous As to adsorbed As and the transformation of Fe minerals, which act as sorption hosts, from amorphous to more stable phases. The developed model provides the basis for predictions of the remedial benefits of Fe(II) nitrate treatments under varying geochemical and hydrogeological conditions, particularly in high-As coastal environments.
Keywords: arsenic remediation; coastal aquifer; geochemical modeling; in situ mineral transformation.
Conflict of interest statement
Notes The authors declare no competing financial interest.
Figures


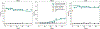


References
-
- Dixit S; Hering JG Comparison of Arsenic(V) and Arsenic(III) Sorption onto Iron Oxide Minerals: Implications for Arsenic Mobility. Environ. Sci. Technol. 2003, 37 (18), 4182–4189. - PubMed
-
- Dregulo A; Bobylev N Heavy Metals and Arsenic Soil Contamination Resulting from Wastewater Sludge Urban Landfill Disposal. Pol. J. Environ. Stud. 2021, 30 (1), 81–89.
-
- Prommer H; Sun J; Helm L; Rathi B; Siade AJ; Morris R Deoxygenation Prevents Arsenic Mobilization during Deepwell Injection into Sulfide-Bearing Aquifers. Environ. Sci. Technol. 2018, 52 (23), 13801–13810. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
