Master equation modeling of water dissociation in small ionic water clusters: Ag+(H2O) n , n = 4-6
- PMID: 39005253
- PMCID: PMC11244579
- DOI: 10.1039/d4ra03518f
Master equation modeling of water dissociation in small ionic water clusters: Ag+(H2O) n , n = 4-6
Abstract
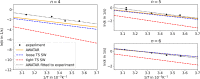
We model temperature-dependent blackbody infrared radiative dissociation (BIRD) rate coefficients of Ag+(H2O) n , n = 4-6, a system with loosely bound water molecules. We employ a master equation modeling (MEM) approach with consideration of absorption and emission of blackbody radiation, comparing single and multiple-well descriptions. The unimolecular dissociation rate coefficients are obtained using the Rice-Ramsperger-Kassel-Marcus (RRKM) theory, employing two approaches to model the sum of states in the transition state, the rigid activated complex (RAC) and the phase space limit (PSL) approach. A genetic algorithm is used to find structures of low-lying isomers for the kinetic modeling. We show that the multiple-well MEM approach with PSL RRKM in the All Wells and Transition States Are Relevant (AWATAR) variant provides a reliable description of Ag+(H2O) n BIRD, in agreement with previously published experimental data. Higher-lying isomers contribute significantly to the overall dissociation rate coefficient, underlying the importance of the multiple-well ansatz in which all isomers are treated on the same footing.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




Similar articles
-
Simplified Multiple-Well Approach for the Master Equation Modeling of Blackbody Infrared Radiative Dissociation of Hydrated Carbonate Radical Anions.J Am Chem Soc. 2022 Nov 30;144(47):21485-21493. doi: 10.1021/jacs.2c07060. Epub 2022 Nov 16. J Am Chem Soc. 2022. PMID: 36383735 Free PMC article.
-
Master equation modeling of blackbody infrared radiative dissociation (BIRD) of hydrated peroxycarbonate radical anions.J Chem Phys. 2024 Apr 7;160(13):134304. doi: 10.1063/5.0200253. J Chem Phys. 2024. PMID: 38557850
-
Water binding energies of [Pb(amino acid-H)H2O]+ complexes determined by blackbody infrared radiative dissociation.Phys Chem Chem Phys. 2012 Nov 21;14(43):15118-26. doi: 10.1039/c2cp41440f. Epub 2012 Oct 5. Phys Chem Chem Phys. 2012. PMID: 23041843
-
Magic cluster sizes of cationic and anionic sodium chloride clusters explained by statistical modeling of the complete phase space.Phys Chem Chem Phys. 2024 Apr 3;26(14):10904-10918. doi: 10.1039/d4cp00357h. Phys Chem Chem Phys. 2024. PMID: 38525830 Free PMC article.
-
Dissociation of 1,1,1-trifluoroethane is an intrinsic RRKM process: classical trajectories and successful master equation modeling.J Phys Chem A. 2015 Mar 12;119(10):1846-58. doi: 10.1021/acs.jpca.5b00796. Epub 2015 Feb 18. J Phys Chem A. 2015. PMID: 25664485
Cited by
-
Electron attachment to CH3COCl molecule and clusters.RSC Adv. 2025 Jul 10;15(29):23983-23993. doi: 10.1039/d5ra02679b. eCollection 2025 Jul 4. RSC Adv. 2025. PMID: 40642467 Free PMC article.
References
-
- Duncan M. A. Spectroscopy of Metal Ion Complexes: Gas-Phase Models for Solvation. Annu. Rev. Phys. Chem. 1997;48:69–93. - PubMed
-
- Duncan M. A. Infrared Spectroscopy to Probe Structure and Dynamics in Metal Ion-Molecule Complexes. Int. Rev. Phys. Chem. 2003;22:407–435.
-
- Beyer M. K. Hydrated Metal Ions in the Gas Phase. Mass Spectrom. Rev. 2007;26:517–541. - PubMed
-
- Ke H. van der Linde C. Lisy J. M. Insights into gas-phase structural conformers of hydrated rubidium and cesium cations, M+(H2O)nAr (M = Rb, Cs; n = 3-5), using infrared photodissociation spectroscopy. J. Phys. Chem. A. 2014;118:1363–1373. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

