This is a preprint.
IL-13 decreases susceptibility to airway epithelial SARS-CoV-2 infection but increases disease severity in vivo
- PMID: 39005257
- PMCID: PMC11244965
- DOI: 10.1101/2024.07.03.601941
IL-13 decreases susceptibility to airway epithelial SARS-CoV-2 infection but increases disease severity in vivo
Abstract
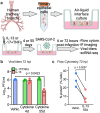
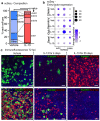
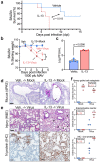
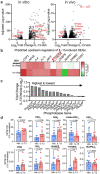
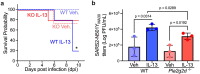
Treatments available to prevent progression of virus-induced lung diseases, including coronavirus disease 2019 (COVID-19) are of limited benefit once respiratory failure occurs. The efficacy of approved and emerging cytokine signaling-modulating antibodies is variable and is affected by disease course and patient-specific inflammation patterns. Therefore, understanding the role of inflammation on the viral infectious cycle is critical for effective use of cytokine-modulating agents. We investigated the role of the type 2 cytokine IL-13 on SARS-CoV-2 binding/entry, replication, and host response in primary HAE cells in vitro and in a model of mouse-adapted SARS-CoV-2 infection in vivo. IL-13 protected airway epithelial cells from SARS-CoV-2 infection in vitro by decreasing the abundance of ACE2-expressing ciliated cells rather than by neutralization in the airway surface liquid or by interferon-mediated antiviral effects. In contrast, IL-13 worsened disease severity in mice; the effects were mediated by eicosanoid signaling and were abolished in mice deficient in the phospholipase A2 enzyme PLA2G2D. We conclude that IL-13-induced inflammation differentially affects multiple steps of COVID-19 pathogenesis. IL-13-induced inflammation may be protective against initial SARS-CoV-2 airway epithelial infection; however, it enhances disease progression in vivo. Blockade of IL-13 and/or eicosanoid signaling may be protective against progression to severe respiratory virus-induced lung disease.
Keywords: COVID-19; IL-13; SARS-CoV-2; eicosanoids; prostaglandin; scRNA-seq.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures

Similar articles
-
SARS-CoV-2 infection of airway cells causes intense viral and cell shedding, two spreading mechanisms affected by IL-13.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2119680119. doi: 10.1073/pnas.2119680119. Epub 2022 Mar 30. Proc Natl Acad Sci U S A. 2022. PMID: 35353667 Free PMC article.
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Long-Term Modeling of SARS-CoV-2 Infection of In Vitro Cultured Polarized Human Airway Epithelium.mBio. 2020 Nov 6;11(6):e02852-20. doi: 10.1128/mBio.02852-20. mBio. 2020. PMID: 33158999 Free PMC article.
-
Molecular Pathogenesis, Immunopathogenesis and Novel Therapeutic Strategy Against COVID-19.Front Mol Biosci. 2020 Aug 11;7:196. doi: 10.3389/fmolb.2020.00196. eCollection 2020. Front Mol Biosci. 2020. PMID: 32850977 Free PMC article. Review.
-
Type 2 Immunity and Its Impact on COVID-19 Infection in the Airways.Viruses. 2023 Jan 31;15(2):402. doi: 10.3390/v15020402. Viruses. 2023. PMID: 36851616 Free PMC article. Review.
References
-
- Matthay MA, and Luetkemeyer AF. IL-6 Receptor Antagonist Therapy for Patients Hospitalized for COVID-19: Who, When, and How? JAMA. 2021;326(6):483–5. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
