This is a preprint.
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation
- PMID: 39005295
- PMCID: PMC11244939
- DOI: 10.1101/2024.07.01.601587
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation
Update in
-
CRISPR-GEM: A Novel Machine Learning Model for CRISPR Genetic Target Discovery and Evaluation.ACS Synth Biol. 2024 Oct 18;13(10):3413-3429. doi: 10.1021/acssynbio.4c00473. Epub 2024 Oct 7. ACS Synth Biol. 2024. PMID: 39375864 Free PMC article.
Abstract
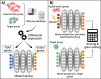
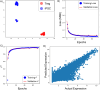
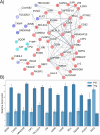
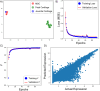
CRISPR gene editing strategies are shaping cell therapies through precise and tunable control over gene expression. However, achieving reliable therapeutic effects with improved safety and efficacy requires informed target gene selection. This depends on a thorough understanding of the involvement of target genes in gene regulatory networks (GRNs) that regulate cell phenotype and function. Machine learning models have been previously used for GRN reconstruction using RNA-seq data, but current techniques are limited to single cell types and focus mainly on transcription factors. This restriction overlooks many potential CRISPR target genes, such as those encoding extracellular matrix components, growth factors, and signaling molecules, thus limiting the applicability of these models for CRISPR strategies. To address these limitations, we have developed CRISPR-GEM, a multi-layer perceptron (MLP)-based synthetic GRN constructed to accurately predict the downstream effects of CRISPR gene editing. First, input and output nodes are identified as differentially expressed genes between defined experimental and target cell/tissue types respectively. Then, MLP training learns regulatory relationships in a black-box approach allowing accurate prediction of output gene expression using only input gene expression. Finally, CRISPR-mimetic perturbations are made to each input gene individually and the resulting model predictions are compared to those for the target group to score and assess each input gene as a CRISPR candidate. The top scoring genes provided by CRISPR-GEM therefore best modulate experimental group GRNs to motivate transcriptomic shifts towards a target group phenotype. This machine learning model is the first of its kind for predicting optimal CRISPR target genes and serves as a powerful tool for enhanced CRISPR strategies across a range of cell therapies.
Figures
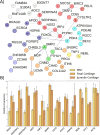
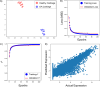
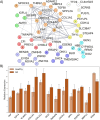
References
-
- Graham J., Werba L., Federico I. & Gonzalez-Fernandez T. CRISPR Strategies for Stem Cell Engineering: A New Frontier in Musculoskeletal Regeneration. Eur. Cell. Mater. 46, 91–118 (2023).
-
- Yoshikawa T. et al. Genetic Ablation of PRDM1 in Antitumor T Cells Enhances Therapeutic Efficacy of Adoptive Immunotherapy. Blood 139, 2156–2172 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
