This is a preprint.
Glia control experience-dependent plasticity in an olfactory critical period
- PMID: 39005309
- PMCID: PMC11245089
- DOI: 10.1101/2024.07.05.602232
Glia control experience-dependent plasticity in an olfactory critical period
Update in
-
Glia control experience-dependent plasticity in an olfactory critical period.Elife. 2025 Jan 30;13:RP100989. doi: 10.7554/eLife.100989. Elife. 2025. PMID: 39883485 Free PMC article.
Abstract
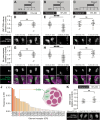
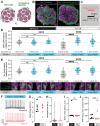
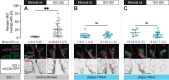
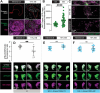
Sensory experience during developmental critical periods has lifelong consequences for circuit function and behavior, but the molecular and cellular mechanisms through which experience causes these changes are not well understood. The Drosophila antennal lobe houses synapses between olfactory sensory neurons (OSNs) and downstream projection neurons (PNs) in stereotyped glomeruli. Many glomeruli exhibit structural plasticity in response to early-life odor exposure, indicating a general sensitivity of the fly olfactory circuitry to early sensory experience. We recently found that glia shape antennal lobe development in young adults, leading us to ask if glia also drive experience-dependent plasticity during this period. Here we define a critical period for structural and functional plasticity of OSN-PN synapses in the ethyl butyrate (EB)-sensitive glomerulus VM7. EB exposure for the first two days post-eclosion drives large-scale reductions in glomerular volume, presynapse number, and post-synaptic activity. Crucially, pruning during the critical period has long-term consequences for circuit function since both OSN-PN synapse number and spontaneous activity of PNs remain persistently decreased following early-life odor exposure. The highly conserved engulfment receptor Draper is required for this critical period plasticity as ensheathing glia upregulate Draper, invade the VM7 glomerulus, and phagocytose OSN presynaptic terminals in response to critical-period EB exposure. Loss of Draper fully suppresses the morphological and physiological consequences of critical period odor exposure, arguing that phagocytic glia engulf intact synaptic terminals. These data demonstrate experience-dependent pruning of synapses and argue that Drosophila olfactory circuitry is a powerful model for defining the function of glia in critical period plasticity.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
