This is a preprint.
Microglia Morphological Response to Mesenchymal Stromal Cell Extracellular Vesicles Demonstrates EV Therapeutic Potential for Modulating Neuroinflammation
- PMID: 39005342
- PMCID: PMC11245023
- DOI: 10.1101/2024.07.01.601612
Microglia Morphological Response to Mesenchymal Stromal Cell Extracellular Vesicles Demonstrates EV Therapeutic Potential for Modulating Neuroinflammation
Update in
-
Microglia morphological response to mesenchymal stromal cell extracellular vesicles demonstrates EV therapeutic potential for modulating neuroinflammation.J Biol Eng. 2024 Oct 17;18(1):58. doi: 10.1186/s13036-024-00449-w. J Biol Eng. 2024. PMID: 39420399 Free PMC article.
Abstract
Background: Mesenchymal stromal cell derived extracellular vesicles (MSC-EVs) are a promising therapeutic for neuroinflammation. MSC-EVs can interact with microglia, the resident immune cells of the brain, to exert their immunomodulatory effects. In response to inflammatory cues, such as cytokines, microglia undergo phenotypic changes indicative of their function e.g. morphology and secretion. However, these changes in response to MSC-EVs are not well understood. Additionally, no disease-relevant screening tools to assess MSC-EV bioactivity exist, which has further impeded clinical translation. Here, we developed a quantitative, high throughput morphological profiling approach to assess the response of microglia to neuroinflammation-relevant signals and whether this morphological response can be used to indicate the bioactivity of MSC-EVs.
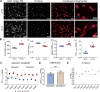
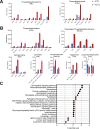
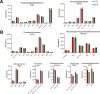
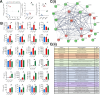
Results: Using an immortalized human microglia cell-line, we observed increased size (perimeter, major axis length) and complexity (form factor) upon stimulation with interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). Upon treatment with MSC-EVs, the overall morphological score (determined using principal component analysis) shifted towards the unstimulated morphology, indicating that MSC-EVs are bioactive and modulate microglia. The morphological effects of MSC-EVs in TNF-γ/IFN-α stimulated cells were concomitant with reduced secretion of 14 chemokines/cytokines (e.g. CXCL6, CXCL9) and increased secretion of 12 chemokines/cytokines (e.g. CXCL8, CXCL10). Proteomic analysis of cell lysates revealed significant increases in 192 proteins (e.g. HIBADH, MEAK7, LAMC1) and decreases in 257 proteins (e.g. PTEN, TOM1, MFF) with MSC-EV treatment. Of note, many of these proteins are involved in regulation of cell morphology and migration. Gene Set Variation Analysis revealed upregulation of pathways associated with immune response, such as regulation of cytokine production, immune cell infiltration (e.g. T cells, NK cells) and morphological changes (e.g. Semaphorin, RHO/Rac signaling). Additionally, changes in microglia mitochondrial morphology were measured suggesting that MSC-EV modulate mitochondrial metabolism.
Conclusion: This study comprehensively demonstrates the effects of MSC-EVs on human microglial morphology, cytokine secretion, cellular proteome, and mitochondrial content. Our high-throughput, rapid, low-cost morphological approach enables screening of MSC-EV batches and manufacturing conditions to enhance EV function and mitigate EV functional heterogeneity in a disease relevant manner. This approach is highly generalizable and can be further adapted and refined based on selection of the disease-relevant signal, target cell, and therapeutic product.
Keywords: Mesenchymal stromal cell-derived extracellular vesicles (MSC-EVs); Microglia; lipidomics; mitochondria; morphology; neuroinflammation; proteomics; secretion.
Conflict of interest statement
Competing Interests The authors have no competing interests to declare. The views presented in this article do not necessarily reflect the current or future opinion or policy of the US Food and Drug Administration.
Figures



References
-
- Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Vol. 164, Cell. Cell Press; 2016. p. 1226–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
