This is a preprint.
APCCdh1-mediated degradation of Cdh1 is necessary for faithful meiotic chromosome segregation in S. cerevisiae
- PMID: 39005361
- PMCID: PMC11245022
- DOI: 10.1101/2024.07.01.601619
APCCdh1-mediated degradation of Cdh1 is necessary for faithful meiotic chromosome segregation in S. cerevisiae
Abstract
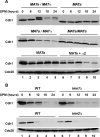
The Anaphase-Promoting Complex/Cyclosome (APC/C) is a ubiquitin ligase that promotes the ubiquitination and subsequent degradation of numerous cell cycle regulators during mitosis and in G1. Proteins are recruited to the APC/C by activator proteins such as Cdh1. During the cell cycle, Cdh1 is subject to precise regulation so that substrates are not degraded prematurely. We have explored the regulation of Cdh1 during the developmental transition into meiosis and sporulation in the budding yeast S. cerevisiae. Transition to sporulation medium triggers the degradation of Cdh1. Cdh1 degradation is mediated by the APC/C itself in a "trans" mechanism in which one molecule of Cdh1 recruits a second molecule of Cdh1 to the APC/C for ubiquitination. Degradation requires an intact glucose-sensing SNF1 protein kinase complex (orthologous to the mammalian AMPK nutritional sensor), which directly phosphorylates Cdh1 on Ser-200 within an unstructured N-terminal region. In the absence of phosphorylation, expression of a Cdh1-S200A mutant is fully stabilized, leading to chromosome instability and loss of viability. We hypothesize that Cdh1 degradation is necessary for the preservation of cell cycle regulators and chromosome cohesion proteins between the reductional and equational meiotic divisions, which occur without the intervening Gap or S phases found in mitotic cell cycles.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
