An Efficient Synthesis of Novel Aminothiazolylacetamido-Substituted 3,5-Bis(arylidene)-4-piperidone Derivatives and Their Cytotoxicity Studies
- PMID: 39005779
- PMCID: PMC11238287
- DOI: 10.1021/acsomega.4c00039
An Efficient Synthesis of Novel Aminothiazolylacetamido-Substituted 3,5-Bis(arylidene)-4-piperidone Derivatives and Their Cytotoxicity Studies
Abstract
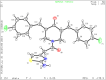
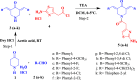
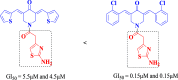
The expansion of 3,5-bis(arylidene)-4-piperidone derivatives with heterocyclic compounds such as 1,3-thiazole should take into account this correlation. The synthesized aminothiazolylacetamido-substituted 3,5-bis(arylidene)-4-piperidone derivatives 3a-j were found to have GI50 values in the range of 0.15-0.28 μM against HeLa and HCT116 cancer cell lines. In silico docking studies confirmed that the proteasome inhibition mechanism involves a nucleophilic attack from the N-terminal threonine residue of the β-subunits to the C=O group of compounds. A C=O group of amide was able to interact with the NH group of the alanine residue and the 5g NH group of amino thiazole, along with an OH group of the serine residue. These results strongly suggest that the synthesized compounds could be a potential candidate inhibitor of the 20S proteasome. These molecules have the potential to be developed as cytotoxic and anticancer agents, as revealed by this study.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- ME J. F.; Siegel R. L.; Isabelle Soerjomataram M. D.; Ahmedin Jemal D. V. M.. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2024. - PubMed
-
- Reed S. I.The ubiquitin-proteasome pathway in cell cycle control. In Cell Cycle Regulation; Springer, 2006; pp 147–181. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
