Interaction of Serum and Plasma Proteins with Polyelectrolyte Microparticles with Core/Shell and Shell-Only Structures
- PMID: 39005812
- PMCID: PMC11238302
- DOI: 10.1021/acsomega.4c03307
Interaction of Serum and Plasma Proteins with Polyelectrolyte Microparticles with Core/Shell and Shell-Only Structures
Abstract
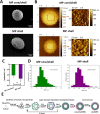
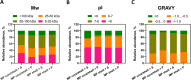
Polyelectrolyte microparticles (MPs) synthesized on calcium carbonate cores are considered a promising basis for new drug delivery systems. It is known that microparticles entering a physiological environment absorb proteins on their surface, which can change the properties of the microparticles and alter their functional activity. This study aimed to compare the compositions of the adsorbed protein layer formed on microparticles with the core/shell and shell structures obtained by layer-by-layer deposition. The difference in the microparticle structure was associated with changes in their surface topography and ζ-potential. These microparticles were incubated with human serum or plasma at 37°C for 24 h. The adsorbed proteins were eluted and analyzed by means of SDS-PAGE. The protein composition of the eluates was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS); a total of 357 proteins were identified, and 183 of them were detected in all samples. Our results demonstrate that the relative abundance of proteins of different functional groups (immunoglobulins, complement proteins, and apolipoproteins) varied depending on the structure and surface characteristics of the polyelectrolyte microparticles and the incubation medium. Our findings expand the understanding of the influence of the physicochemical properties of the microparticles on their interaction with proteins, which can help to improve the design of microparticles for drug delivery.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Galogahi F. M.; Zhu Y.; An H.; Nguyen N. T. Core-Shell Microparticles: Generation Approaches and Applications. J. Sci.: Adv. Mater. Devices 2020, 5, 417–435. 10.1016/j.jsamd.2020.09.001. - DOI
LinkOut - more resources
Full Text Sources
