The usefulness of peri-trigger female reproductive hormones (delta-FRH) in predicting oocyte maturation in normal ovarian reserve patients who received in vitro fertilization-embryo transfer: a retrospective study
- PMID: 39006021
- PMCID: PMC11246619
- DOI: 10.7717/peerj.17706
The usefulness of peri-trigger female reproductive hormones (delta-FRH) in predicting oocyte maturation in normal ovarian reserve patients who received in vitro fertilization-embryo transfer: a retrospective study
Abstract
Objectives: To evaluate the efficacy of peri-trigger female reproductive hormones (FRHs) in the prediction of oocyte maturation in normal ovarian reserve patients during the in vitro fertilization-embryo transfer (IVF-ET) procedure.
Materials and methods: A hospital database was used to extract data on IVF-ET cases from January 2020 to September 2021. The levels of female reproductive hormones, including estradiol (E2), luteinizing hormone (LH), progesterone (P), and follicle-stimulating hormone (FSH), were initially evaluated at baseline, the day of the trigger, the day after the trigger, and the day of oocyte retrieval. The relative change in E2, LH, P, FSH between time point 1 (the day of trigger and baseline) and time point 2 (the day after the trigger and day on the trigger) was defined as E2_RoV1/2, LH_RoV1/2, P_RoV1/2, and FSH_RoV1/2, respectively. Univariable and multivariable regression were performed to screen the peri-trigger FRHs for the prediction of oocyte maturation.
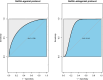
Results: A total of 118 patients were enrolled in our study. Univariable analysis revealed significant associations between E2_RoV1 and the rate of MII oocytes in the GnRH-agonist protocol group (p < 0.05), but not in the GnRH-antagonist protocol group. Conversely, P_RoV2 emerged as a potential predictor for the rate of MII oocytes in both protocol groups (p < 0.05). Multivariable analysis confirmed the significance of P_RoV2 in predicting oocyte maturation rate in both groups (p < 0.05), while the association of E2_RoV1 was not significant in either group. However, within the subgroup of high P_RoV2 in the GnRH-agonist protocol group, association was not observed to be significant. The C-index was 0.83 (95% CI [0.73-0.92]) for the GnRH-agonist protocol group and 0.77 (95% CI [0.63-0.90]) for the GnRH-antagonist protocol group. The ROC curve analysis further supported the satisfactory performance of the models, with area under the curve (AUC) values of 0.79 for the GnRH-agonist protocol group and 0.81 for the GnRH-antagonist protocol group.
Conclusions: P_RoV2 showed significant predictive value for oocyte maturation in both GnRH-agonist and GnRH-antagonist protocol groups, which enhances the understanding of evaluating oocyte maturation and inform individualized treatment protocols in controlled ovarian hyperstimulation during IVF-ET for normal ovarian reserve patients.
Keywords: Biomarker; Female reproductive hormone; In vitro fertilization-embryo transfer; Oocyte maturity; Peri-trigger management.
©2024 He et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
Is oocyte maturation rate associated with triptorelin dose used for triggering final oocyte maturation in patients at high risk for severe ovarian hyperstimulation syndrome?Hum Reprod. 2019 Sep 29;34(9):1770-1777. doi: 10.1093/humrep/dez105. Hum Reprod. 2019. PMID: 31384921
-
Predicting suboptimal oocyte yield following GnRH agonist trigger by measuring serum LH at the start of ovarian stimulation.Hum Reprod. 2019 Oct 2;34(10):2027-2035. doi: 10.1093/humrep/dez132. Hum Reprod. 2019. PMID: 31560740
-
Early luteal phase endocrine profile is affected by the mode of triggering final oocyte maturation and the luteal phase support used in recombinant follicle-stimulating hormone-gonadotropin-releasing hormone antagonist in vitro fertilization cycles.Fertil Steril. 2013 Sep;100(3):742-7. doi: 10.1016/j.fertnstert.2013.05.028. Epub 2013 Jun 24. Fertil Steril. 2013. PMID: 23806846 Clinical Trial.
-
A study to determine the efficacy of controlled ovarian hyperstimulation regimen using a gonadotropin releasing hormone agonist versus antagonist in women of advanced reproductive age with varying degrees of oocyte reserve on outcome following in vitro fertilization-embryo transfer.Clin Exp Obstet Gynecol. 2013;40(2):191-2. Clin Exp Obstet Gynecol. 2013. PMID: 23971234 Review.
-
Comparison the effects of progestin-primed ovarian stimulation (PPOS) protocol and GnRH-a long protocol in patients with normal ovarian reserve function.Gynecol Endocrinol. 2023 Dec;39(1):2217263. doi: 10.1080/09513590.2023.2217263. Gynecol Endocrinol. 2023. PMID: 37236243 Review.
References
-
- Baldini D, Savoia MV, Sciancalepore AG, Malvasi A, Vizziello D, Beck R, Vizziello G. High progesterone levels on the day of HCG administration do not affect the embryo quality and the reproductive outcomes of frozen embryo transfers. Clinica Terapeutica. 2018;169:e91–e95. doi: 10.7417/T.2018.2060. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

