Coumestrol facilitates apoptosis in colorectal cancer cells by interacting with ZIP8 protein via the ferroptosis pathway
- PMID: 39006076
- PMCID: PMC11242349
- DOI: 10.7150/jca.94628
Coumestrol facilitates apoptosis in colorectal cancer cells by interacting with ZIP8 protein via the ferroptosis pathway
Abstract
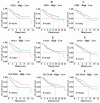
Objective: So far, there have been no reports of coumestrol inhibiting colorectal cancer (CRC) through the ferroptosis pathway. This study is to investigate the mechanism of the traditional Chinese medicine monomer coumestrol in the treatment of CRC. Methods: Data on CRC transcriptome sequencing was obtained from the GEO database and TCGA database. Bioinformatics analyses were conducted to screen for CRC prognostic-related key genes and their potential binding monomers in traditional Chinese medicine. The inhibitory effect of coumestrol on CRC cell lines (COLO 205 & HCT 116) was determined using the CCK-8 assay, and cell apoptosis was assessed by flow cytometry. The content of ferrous ions was measured using the Ferrous Ion Content Assay Kit. The expression of ferroptosis pathway-related genes SLC39A8, NCOA4, VDAC2, and NOX2 before and after small interference RNA (siRNA) was examined through real-time PCR and Western blotting. Results: SLC39A8 was found to be associated with CRC clinical progression staging, and its encoded protein ZIP8 may bind to coumestrol. KEGG enrichment analysis suggested that ZIP8 plays a role in iron transmembrane transport and may affect the expression of ferroptosis pathway-related genes NCOA4, VDAC2, and NOX2. Coumestrol was found to induce apoptosis in CRC cell lines by upregulating the expression of ferroptosis pathway-related genes SLC39A8, NCOA4, VDAC2, and NOX2. However, coumestrol was unable to upregulate the expression of ferroptosis pathway-related genes in CRC cell lines after SLC39A8 interference. Conclusion: Coumestrol facilitates apoptosis in CRC cells by interacting with ZIP8 protein via the ferroptosis pathway.
Keywords: SLC39A8; ZIP8; colorectal cancer; coumestrol; ferroptosis pathway.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures







References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. - PubMed
-
- Buchwald P, Hall C, Davidson C, Dixon L, Dobbs B, Robinson B. et al. Improved survival for rectal cancer compared to colon cancer: the four cohort study. ANZ journal of surgery. 2018;88:E114–E7. - PubMed
-
- Johansen JS, Christensen IJ, Jorgensen LN, Olsen J, Rahr HB, Nielsen KT. et al. Serum YKL-40 in risk assessment for colorectal cancer: a prospective study of 4,496 subjects at risk of colorectal cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:621–6. - PubMed
-
- Roseth AG, Kristinsson J, Fagerhol MK, Schjonsby H, Aadland E, Nygaard K. et al. Faecal calprotectin: a novel test for the diagnosis of colorectal cancer? Scandinavian journal of gastroenterology. 1993;28:1073–6. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

